Журнал "Макрогетероциклы"
Журнал является форумом специалистов, изучающих макрогетероциклические соединения
Navigation
News
Impact Factor 2021 = 1.200 has been issued by ISI Web of Knowledge (JCR 2021).
Search
ISSN 1998-9539
Vol. 2 N 3-4
Preface |
|
Conferences |
|
 The survey is given of the 10th International Conference on Physical and Coordination Chemistry of Porphyrins and Their Analogues (Ivanovo, July 1-4, 2009). The survey is given of the 10th International Conference on Physical and Coordination Chemistry of Porphyrins and Their Analogues (Ivanovo, July 1-4, 2009). |
D. B. Berezin
10th International Conference on Physical and Coordination Chemistry of Porphyrins and Their Analogues (ICPC-10) 187-189 |
Porphyrins |
Microreview |
 Works of authors on synthesis, study of structure and magnetic properties of molecular complexes formed by fullerene anions with metal porphyrins coordinated with one or two N-metyldiazabicyclooctane cations are reviewed. Works of authors on synthesis, study of structure and magnetic properties of molecular complexes formed by fullerene anions with metal porphyrins coordinated with one or two N-metyldiazabicyclooctane cations are reviewed. |
D. V. Konarev, R. N. Lyubovskaya
Coordination structures of Metalloporphyrins with N-Containing Cations in Complexes with Fullerenes 190-197 |
|
Paper |
|
A convenient and straightforward synthetic approach to tetrabenzo- and tetranaphtho¬porphyrins based on 4,7-dihydroisoindole and its benzoannelated derivative was developed.
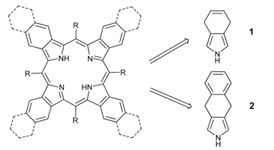 |
M. A. Filatov, S. E. Aleshchenkov, A. V. Cheprakov
A Versatile General Approach to the Synthesis of Linearly Annelated π Extended Porphyrins via 4,7 Dihydroisoindole Derivatives 198-205 |
Porphyrins |
Paper |
|
Self-assembly principles (based on the non-covalent two-fold extra-ligation) elaborated for the formation of porphyrin triads and pentads have been successfully employed to anchor tetrapyrrole molecules on semiconductor CdSe/ZnS quantum dot surfaces in solutions.
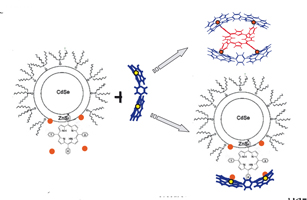 |
E. I. Zenkevich, C. von Borczyskowski
Photoinduced Relaxation Processes in Self-Assembled Nanostructures: Multiporphyrin Complexes and Composites “CdSe/ZnS Quantum Dot -Porphyrin” 206-220 |
Porphyrins |
Paper |
|
Based on amino derivatives of 5,10,15,20-tetraphenylporphyrin and functionally substituted carboranes boronated porphyrins with high boron content (~30-35%) were obtained.
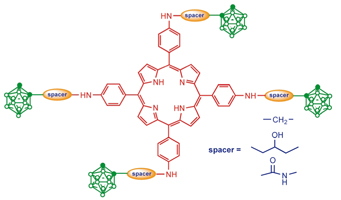 |
V. А. Ol’shevskaya, А. V. Zaitsev, Y. V. Dutikova,, V. N. Luzgina, E. G. Kononova, P. V. Petrovsky, V. N. Kalinin
A One Step Synthesis of Boronated meso-Tetraphenylporphyrins. 221-227 |
|
|
|
We described the synthesis of the series of mesogenic symmetrical 5,15- and 5,10,15,20-meso-arylsubstituted porphyrins containing long chain alkyl and acyl groups which exhibit thermotropic and lyotropic behaviour in nonpolar solvents, some of them being glassing mesogens.
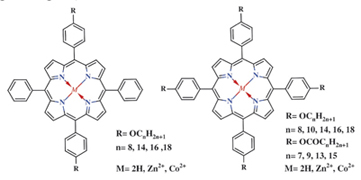 |
N. A. Bragina, I. N. Fedulova, E. S. Krutikova, N. V. Novikov, A. F. Mironov, V. V. Bykova, N. V. Usol’tseva, G. A. Ananieva
Synthesis and Mesogenic Properties of Lipophilic and Amphiphilic Tetraphenylporphyrins 228-236 |
|
|
|
Radical polymerization of methyl metacrylate and styrene in the presence of iron and cobalt complexes of porphyrines have been studied and feasible polymerization scheme was proposed.
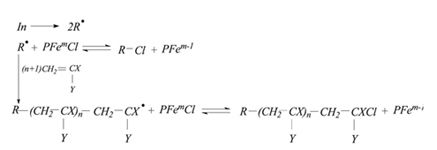 |
Yu. B. Monakov, R. M. Islamova, O. I. Koifman
Complexes of Iron and Cobalt Porphyrins for Controlled Radical Polymerization of Methyl Metacrylate and Styrene 237-242 |
|
|
|
The reactions between the poly(methyl methacrylate) radical and the molecule of chloroiron(III) porphyrin complex have been studied using density functional theory approach.
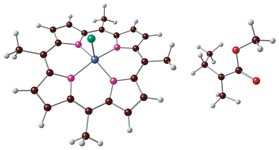 |
A. K. Friesen
DFT Study of the Interaction between Chloroiron(III) Porphyrin and Poly(methylmethacrylate) Macroradical 243-245 |
Porphyrazinoids |
Paper |
|
Alkylation of 2,5-diamino-1,3,4-thiadiazole with alkylbromides leads to 3-alkyl-5-amino-2-imino-1,3,4-thiadiazolines which are condensed with 1,1-dimethoxy-3-iminoisoindoline with formation of new three-units products of ABA-type.
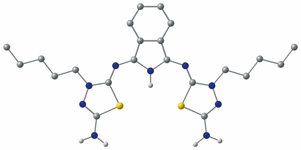 |
E. A. Danilova, T. V. Melenchuk, E. E. Melekhonova, M. A. Tyutina, M. K. Islyaikin
Three-unit Products from Condensation of 3-Alkylated 2,5-Diamino-1,3,4-thiadiazoles with 1,1-Dimethoxy-3-iminoisoindoline 246-250 |
Porphyrins |
Paper |
|
Photophysical properties of the monodeprotonated form of 5,10,15,20-tetrakis(4-N-methylpyridiniumyl)porphyrin have been studied and it has been shown that intersystem crossing is the main pathway for the excitation energy deactivation.
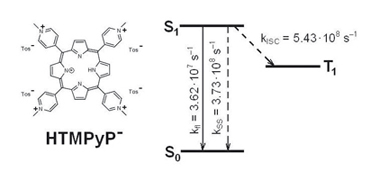 |
M. M. Kruk, A. S. Starukhin
Excitation Energy Deactivation in Monodeprotonated Porphyrin 251-254 |
|
|
|
Planar and saddle-type distorted forms of the Pt-porphin were observed in solid matrices at liquid helium temperature and their optical properties have been studied.
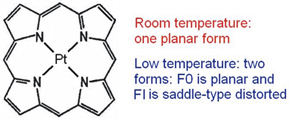 |
A. S. Starukhin, M. M. Kruk
Fine Line Luminescence and Raman Spectra of Distorted Forms of Metalloporphyrins: Pt-porphin 255-257 |
Phthalocyanines |
Communication |
|
The direct synthesis of monohydroxy substituted phthalocyanines has been developed with application of these compounds as building-blocks for selective preparation homo- and heteroligand bis-phthalocyanine complexes.
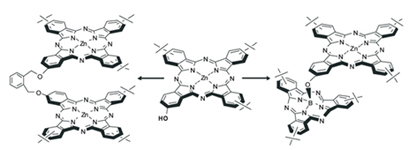 |
A. Yu. Tolbin, L. G. Tomilova
Application of Monohydroxyphthalo¬cyanines for Selective Preparation of Homo- and Heteroligand Macrocyclic Compounds 258-260 |
|
|
|
Monomeric derivative of Fe-phtalocyanine with trimethoxysilyl functional group was attached onto mesoporous molecular sieve SBA-15 and showed higher activity in liquid phase hydroxylation of phenol by hydrogen peroxide than ungrafted Pc analogue.
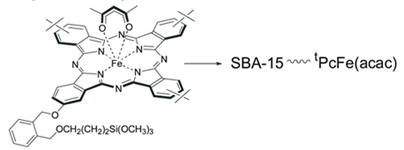 |
A. Yu. Tolbin, S. V. Sirotin, I. F. Moskovskaya, L. G. Tomilova, B. V. Romanovsky
Synthesis of Iron Phthalocyanine Grafted onto SBA-15 through Single Siloxane Bond and Its Application in Liquid-phase Hydroxylation of Phenol 261-263 |
Porphyrins |
Paper |
|
The photocatalytic decomposition of Н2О2 in the presence of chlorophyll and metal porphyrins immobilized on silica was studied. Processes of reduction of some electron acceptors photocatalyzed by chlorophyll in Н2О2 solution are demonstrated.
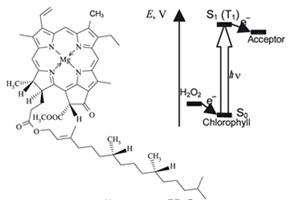 |
O. V. Nevrova, A. V. Lobanov, G. G. Komissarov
Chlorophyll and Metal Porphyrins in Photocatalytic Redox Reactions of Hydrogen Peroxide 264-267 |
Порфирины |
Статья |
|
Influence of different additives on kinetics of hydrogen peroxide decomposition catalyzed by hemin (FePP) and destruction of FePP was studied. Maximum of catalytic activity of FePP in hydrogen peroxide decomposition is shown to correspond to an associated form of FePP.
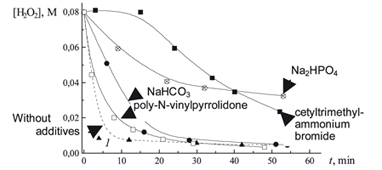 |
A.V. Lobanov, S.M. Vasiliev, G.G. Komissarov
Interaction of Hemin and Hydrogen Peroxide: Effect of Media
268-270 |
Cyclic polyamines |
Paper |
|
Geometric parameters of complexes formed by N,N,N,N-coordinating macroheterocyclic ligand 1,8-dioxa-3,6,10,13-tetraazacyclotetradecanetetrathione-4,5,11,12 with O=VIV, MnII, FeII, CoII, NiII, CuII and ZnII have been calculated using DFT B3LYP method with 6-31G(d) basic set
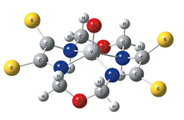 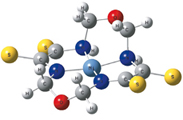 |
D. V. Chachkov, O. V. Mikhailov
DFT Calculations of Space Structures of MII Complexes with (N,N,N,N)-Coordinating Macroheterocyclic Ligand – 1,8-Dioxa-3,6,10,13-tetraaza¬cyclotetradecanetetrathione-4,5,11,12 271-274 |
|
|
|
Pd-catalyzed amination of 3,3'-dibromobiphenyl with various polyamines and oxadiamines afforded a series of novel nitrogen- and oxygen-containing macroheterocycles in yields from moderate to good.
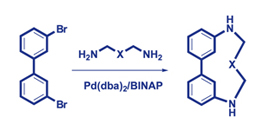 |
A. D. Averin, A. N. Uglov, A. K. Buryak, I. P. Beletskayaa
Facile Synthesis of New Polyazamacrocycles by the Pd-catalyzed Amination of 3,3'-Dibromobiphenyl 275-280 |
|
|
|
Pd-catalyzed amination of 1,3-dibromobenzene with N,N',N''-trimethylcyclam and N,N',N''-trimethylcyclen provided corresponding 1,3-bis(tetraazamacrocyclic) derivatives of benzene in 25-32% yields.
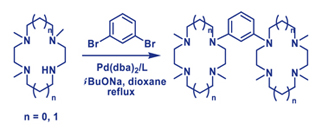 |
A. D. Averin, A. V. Shukhaev, A. K. Buryak, F. Denat, R. Guilard, I. P. Beletskayaa
Synthesis of 1,3-Bis(trimethylcyclam) and 1,3-Bis(trimethylcyclen) Substituted Benzenes 281-285 |
Сrown ethers & Cryptands |
Paper |
|
The synthesis and properties of the first representatives of fluorenonoazacrownophanes, bis(fluorenono)diazacrownophanes and fluorenonocryptand has been described.
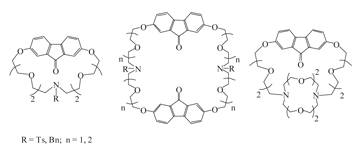 |
A. V. Lobach, I. S. Yakovenko, N. G. Lukyanenko
Synthesis and Properties of the First Representatives of Fluorenonoazacrownophanes and Fluorenonocryptand 286-289 |
Сrown ethers |
|
|
New fluorenocrownophanes containing fragments of 2,7-dioxyfluorene and hydroquinone or 4,4’-dioxybiphenyl linked by oligoethylene glycol residues were synthesized. The formation of the pseudorotaxane type inclusion complexes of these ligands with paraquat was established by means of FAB mass spectrometry, 1H NMR and electronic spectroscopy.
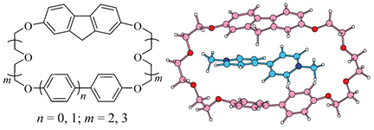 |
T. I. Kirichenko, A. V. Lobach, A. Yu. Lyapunov, C. Yu. Kulygina, I. S. Yakovenko, N. G. Lukyanenko
New Fluorenocrownophanes Containing Benzene or Biphenyl Fragments: Synthesis, Properties and Interaction with Paraquat 290-295 |
- 5676 reads
- Русский
