Журнал "Макрогетероциклы"
Navigation
News
Impact Factor 2021 = 1.200 has been issued by ISI Web of Knowledge (JCR 2021).
Search
ISSN 1998-9539
Vol.1 N.1
|
7-8 |
|
Porphyrinoids |
Review |
|
A review on the synthesis and metalation of carbaporphyrinoid systems is presented. These porphyrin analogues are superior organometallic ligands that are starting to rival the better known N-confused porphyrins
|
T. D. Lash Metalation of Carbaporphyrinoid Systems
9-20 |
Porphyrazines |
Microreview |
|
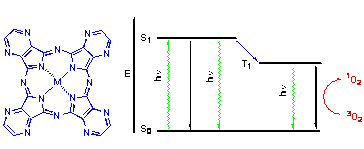
Review summarizes the recent knowledge about the syntheses, spectral properties, aggregation, protonation and deprotonation of macrocycle and singlet oxygen and fluorescence quantum yields of azaphthalocyanines bearing substituted pyrazine rings in their conjugated system.
|
P. Zimcik, V. Novakova, M. Miletin, K. Kopecky
21-29 |
Porphyrazines |
Paper |
|
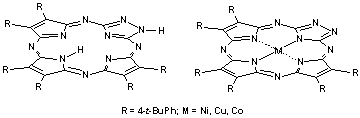
Results of authors’ on the study of triazoleporphyrazines, structural analogues of porphyrazine containing one triazole ring instead of one of pyrrole subunits are summarized.
|
M. K. Islyaikin, O. N. Trukhina, Y. V. Romanenko, E. A. Danilova, O. G. Khelevina
Synthesis, Structure Peculiarities and Acid-Basic Behaviour of Triazoleporphyrazines
30-39 |
Porphyrazines |
Paper |
|
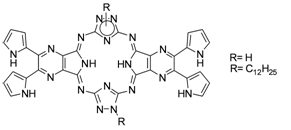
Described in this report is the synthesis and optical spectroscopic characterization of two novel dipyrrolylquinoxaline analogues – triazolehemipyrazinoporphyrazines that are functionalized with four pyrrole rings.
|
M. S. Rodríguez-Morgade, G. D. Pantos, E. Caballero, J. L. Sessler, T. Torres
Synthesis of Hemipyrazinoporphyrazines Bearing Peripheral Pyrrolic Substituent
40-43 |
Phthalocyanines |
Paper |
|
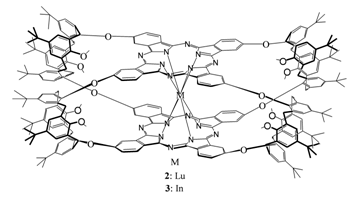
Authors report on synthesis and spectral study of new double-decker phthalocyaninates of lutetium(III) and indium(III) with fourcalix[4]arene bridges |
T. Ceyhan, G. Yağlioğlu, H. Ünver, B. Salih, M. K. Erbil, A. Elmali, Ö. Bekaroğlu
44-49 |
Porphyrins |
Paper |
|
The titration of the diprotonated form of 3,7,13,17-tetramethyl-2,8,12,18-tetrabutylporphyrin with the series of halide ions has been done in the acetonitrile solution and the influence of complexation with iodide ions on the porphyrin fluorescent properties has been studied. |
M. M. Kruk, Yu. B. Ivanova, V. B. Sheinin, A. S. Starukhin, N. Zh. Mamardashvili, O. I Koifman
50-58 |
Porphyrins |
Paper |
|
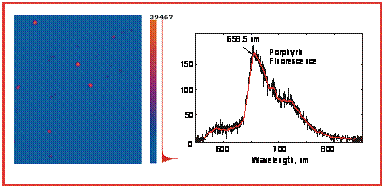
On the basis of laser confocal fluorescence microscopy, ensemble averaged steady-state and kinetic measurements it was shown that closely displaced aminophenyl substituted porphyrins in bright spots on polypropylene film surface do not form aggregates and possess monomeric emission. |
E. I. Zenkevich, J. Martin, C. von Borczyskowski, T. A. Ageeva, V. A Titov, V. N. Knyukshto
59-67 |
Porphyrins |
Paper |
|
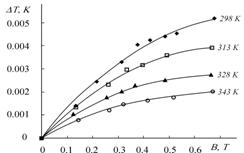
Magnetocaloric effect and specific heat capacity of 6% MnIII octaethyl¬porphyrin suspension in water were measured by microcalorimetric method in the dependence of temperature (298-353 K) and magnetic field (0-0.7 T).
|
V. V. Korolev, M. E. Klyueva, I. M. Arefyev, A. G. Ramazanova, T. N. Lomova, A. G. Zakharov
Regularities of Magnetocaloric Effect and Determination of Some Thermo¬dynamic Parameters for (Octaethyl¬porphynato)chloromanganese(III)
68-71 |
Porphyrins |
Paper |
|
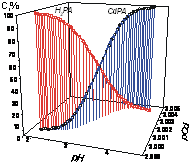
The reactions of metalloporphyrins formation are pH-dependent. Acid-basic properties of porphyrin ligands are changed in wide area and determined the mechanism of complex formation.
|
V. B. Sheinin, O. R. Simonova, E. L. Ratkova
Effect of pH on Formation of Metalloporphyrins
72-78 |
Porphyrins |
Communication |
|
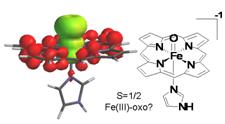
Unexpected electronic structures are unravelled by computational methods for recently-observed ferric-oxo states in hemoproteins.
|
R. Silaghi-Dumitrescu
The Ferric-Oxo Moiety in Porphyrin Complexes – a Ferryl in Disguise?
79-81 |
Porphyrazines |
Communication |
|
Attempt to obtain imidazoporphyrazine by deselenation of 1,2,5-selenadiazoloporphyrazine and subsequent treatment of “diaminoporphyrazine” with formic acid unexpectedly led to diformamidoporphyrazine.
|
A. Ul-Haq, P. A. Stuzhin
Conversion of 1,2,5-Selenadiazoloporphyrazine to Diformamidoporphyrazine
82-84 |
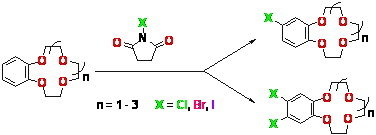
Crown-ethers |
Paper |
|
Halogenation of benzo- and dibenzocrown ethers with N-halogenosuccinimides in solid phase, water or organic medium was studied.
|
S. A. Kotlyar, S. M. Pluzhnik-Gladyr
The Peculiarities of the Reaction of Benzo- and Dibenzocrown Ethers with N-Halogenosuccinimides
85-89 |
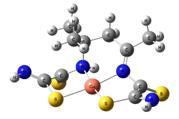
Template synthesis |
Paper |
|
Template synthesis processes for the triple systems MII ion – ambidentate (N,S)-,(N,O,S)-synthon– (C=O)-synthon (M= Co, Ni, Cu) in the solutions and in gelatin-immobilized matrix were studied and analyzed.
|
O. V. Mikhailov, M. A. Kazymova, D. V. Chachkov
Template Synthesis into Gelatin-Immobilized Matrix as Perspective Method of Obtaining Supramolecular Macroheterocyclic Compounds
90-97 |
| Attachment | Size |
|---|---|
| MHC2008_1_page1-29.pdf | 1.37 MB |
| MHC2008_1_page30-67.pdf | 1.9 MB |
| MHC2008_1_page68-100.pdf | 1.87 MB |