Журнал "Макрогетероциклы"
Navigation
News
Impact Factor 2021 = 1.200 has been issued by ISI Web of Knowledge (JCR 2021).
Search
ISSN 1998-9539
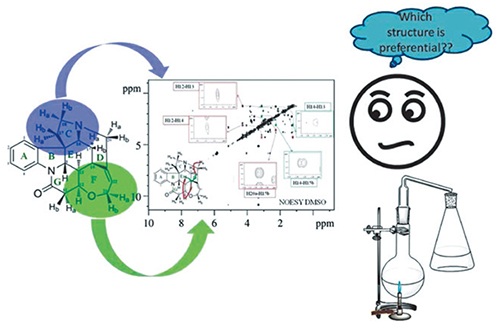
The Spatial Structure of Macroheterocyclic Compounds, as a Key Factor Affecting the Course of the Macrocyclization Reaction
Nowadays the macrocyclization reaction is often used for the synthesis of new macroheterocyclic compounds. This is a one-pot reaction, carried out in one stage, which makes it the most convenient and efficient in the synthesis of complex macroheterocyclic compounds. One of the key parameters in the selection of synthesis conditions is the spatial structure and conformational composition of the synthesized compounds. The fact is that if the certain structure prevails in the solution, then it is the most energetically favorable, i.e. its potential energy is minimal and therefore it is necessary to spend less energy to synthesis of this compound. The structure, obtained during the synthesis process, largely depends on what solvent is used. In this work, it was shown that the conformational composition of the macroheterocyclic model compound in various solvents differs by 10–20 %. This fact must be taken into account when conducting macrocyclization reactions not only for the model compound presented in this paper, but also for other substances consisting of macroheterocyclic molecules, such as crown ethers, cyclosporine, calixarenes, and other classes of compounds having a non-rigid structure.
| Attachment | Size |
|---|---|
| mhc200388k.pdf | 2.2 MB |
| mhc200388k_supp.pdf | 5.37 MB |
- 1395 reads
- Русский
