Журнал "Макрогетероциклы"
Журнал является форумом специалистов, изучающих макрогетероциклические соединения
Navigation
News
Impact Factor 2021 = 1.200 has been issued by ISI Web of Knowledge (JCR 2021).
Search
ISSN 1998-9539
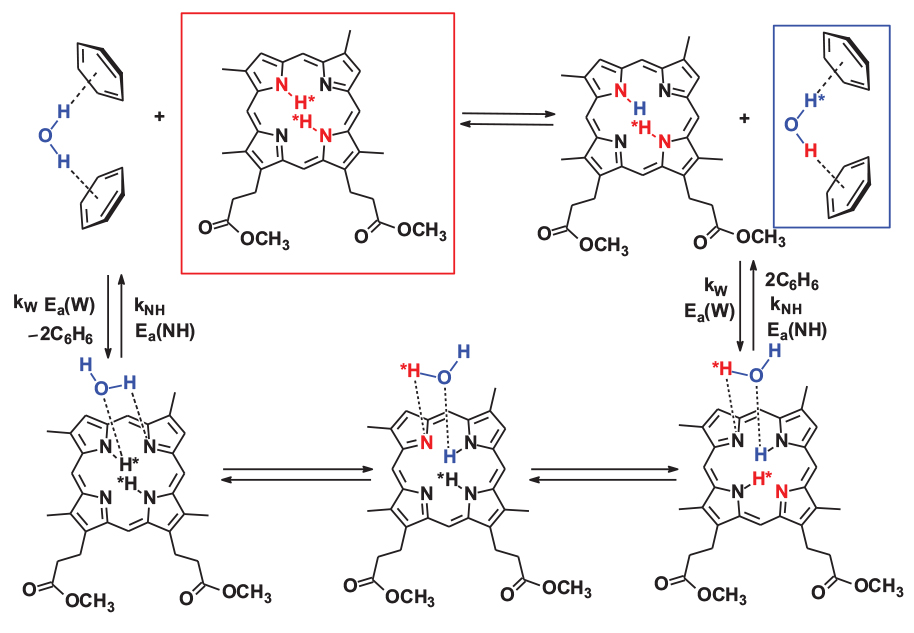
Activation Energy of Proton Exchange Processes of Deuteroporphyrin IX Dimethyl Ester with Water in C6D6 Medium Based on DOSY Data
Alexander L. Stolypkoa and Dmitriy V. Belykh b @
aSyktyvkar State University, 167000 Syktyvkar, Russia
bInstitute of Сhemistry of Komi Scientific Centre of the Ural Branch of the Russian Academy of Sciences, 167000 Syktyvkar, Russia
@Corresponding author E-mail: belykh-dv@mail.ru
DOI: 10.6060/mhc191280b
Macroheterocycles 2019 12(4) 398-402
The activation energies of the processes which determine the change in the self-diffusion coefficient D of water protons and the internal NH protons of deuteroporphyrin IX dimethyl ester (DW and DNH ) associated with the proton exchange between the molecules of these compounds in C6D6 medium are determined by the DOSY method. The activation energies of the processes that cause the change in DW and DNH were estimated to be Ea(W) = 27.3 ± 1.3 kJ/mol and Ea(NH) = 15.8 ± 0.7 kJ/mol, respectively. The data obtained show, that activation barriers in all cases are associated with the breaking of hydrogen bonds during the formation and destruction of porphyrin-water associates, the formation of which is a necessary condition for the exchange.
| Attachment | Size |
|---|---|
| mhc191280b.pdf | 658.97 KB |
- 1306 reads
- Русский
