Журнал "Макрогетероциклы"
Navigation
News
Impact Factor 2021 = 1.200 has been issued by ISI Web of Knowledge (JCR 2021).
Search
ISSN 1998-9539
Synthesis, Aggregation Behavior, and Catalytic Activity in the Ullmann Reaction of Amphiphilic p-tert-Butylthiacalix[4]arene with Azidoalkylimidazolium Moieties
V. A. Burilov,a@ R. I. Garipova,a E. D. Sultanova,a D. A. Mironova,a V. G. Evtugyn,a Yu. N. Osin,a S. E. Solovieva,b I. S. Antipina
aKazan Federal University, 420008 Kazan, Russian Federation
bArbuzov Institute of Organic and Physical Chemistry, FRC Kazan Scientific Center of RAS, 420088 Kazan, Russian Federation
@Corresponding author E-mail: ultrav@bk.ru
Macroheterocycles 2019 12(4) 340-345
DOI: 10.6060/mhc191174b
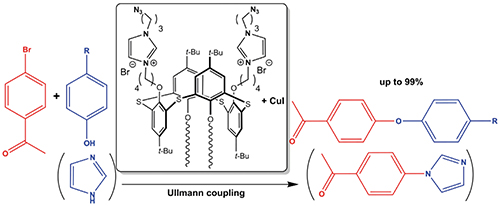
New effective methods for creating carbon-carbon or carbon-heteroatom bonds are a significant task both from a fundamental and practical points of view. Metal complexes with N-heterocyclic carbene (NHC) ligands, being resistant to air oxygen and moisture, are one of the most effective catalysts for coupling reactions, gradually replacing traditional systems based on phosphine metal complexes. Decoration of appropriate carriers with NHC complexes allows to combine the homogeneous and heterogeneous catalysis, obtaining reusable highly active catalytic systems. For the synthesis of such catalytic systems, the necessary anchor groups are introduced into the metal complex or NHC ligand for immobilization on a support having inorganic/organic nature. We propose an absolutely different approach to the formation of supported NHC, which consists in sequential self-assembly of amphiphilic macrocycles containing azidoalkyl fragments in the polar head group into a colloidal nanoparticle in an aqueous solution, followed by covalent fixation of macrocycles by a low molecular weight water-soluble acetylene-containing spacer using copper catalyzed azide-alkine cycloaddition (CuAAC) reaction, which allows controlling the morphology of the obtained support and give opportunity to introduce various NHC fragments both in macrocycle and spacer. To implement the proposed approach, the key step is the synthesis of amphiphilic macrocycles containing azide fragments necessary for CuAAC and capable of forming stable colloids in water. In this regard, the presented work is devoted to the synthesis of the amphiphilic derivative of p-tert-butylthiacalix[4]arene containing charged imidazolium and azidoalkyl fragments, as well as the study of its aggregation in aqueous solutions and catalytic activity in model Ullmann N- and O-arylation. Reaction of p-tert-butylthiacalix[4]arene containing two butyl and two bromobutyl substituents with 3-azidopropylimidazole given amphiphilic bifunctional derivative of p-tert-butylthiacalix[4]arene containing azidoalkyl and imidazolium fragments in the 1,3-alternate stereoisomeric form. It was found that keeping the reaction in toluene is most convenient since the target product falls out of the reaction medium, which allows the use of a small excess of 3-azidopropylimidazole. The structure of the obtained macrocycle was established using a complex of modern physical methods, and the composition was determined by elemental analysis. Due to the presence of charged fragments on the upper rim and small lipophilic fragments on the lower rim, the resulting macrocycle dissolves in water. The critical aggregation concentration (CAC) of the macrocycle in water was studied by the fluorescent probe (pyrene), and was found to be 0.97 mM. The formation of calixarene aggregates in water was confirmed by dynamic and electrophoretic light scattering methods: the formation of stable nanoscale aggregates with an average hydrodynamic diameter of about 110–150 nm begins at concentrations close to CAC. The high positive zeta potential (+39) indicates the stability of the formed aggregates. The morphology of the aggregates transferred from an aqueous solution onto a carbon-sprayed copper mesh plate was also studied. Micrographs show spherical aggregated particles with sizes of about 30–90 nm. Using energy dispersive X-ray spectroscopy, a qualitative elemental composition of the observed aggregates was studied: signals of carbon, sulfur, oxygen, and bromine were detected in the sample, which is consistent with the composition of the studied compound. Given that the copper NHC complexes, including ones obtained in situ from appropriate imidazolium salt and CuI, exhibit high catalytic activity in the Ullmann reaction, we studied the catalytic activity of the CuI in the presence of calixarene in the coupling reaction of p-bromoacetophenone with imidazole and some phenols. According to the data obtained, the reaction of p-bromoacetophenone with imidazole using only CuI given only 58 % of conversion, while addition of macrocycle to CuI significantly increased the conversion to 82 %, which exceeds the activity of catalytic systems known in the literature. The combination of CuI with calixarene was also effective in the Ullmann О-arylation of several substituted phenols.
| Attachment | Size |
|---|---|
| mhc191174b.pdf | 1.23 MB |
- 1168 reads
- Русский
