Журнал "Макрогетероциклы"
Журнал является форумом специалистов, изучающих макрогетероциклические соединения
Navigation
News
Impact Factor 2021 = 1.200 has been issued by ISI Web of Knowledge (JCR 2021).
Search
ISSN 1998-9539
Molecular Structure of 1,2,5-Selenadiazolodibenzosubporphyrazinatoboron(III) Chloride and Influence of Perfluorination and Perchlorination on Its Spectral Properties
Mahmoud Hamdoush,a Nikolay V. Somov,b Svetlana S. Ivanova,a and Pavel A. Stuzhina@
aResearch Institute of Macroheterocycles, Ivanovo State University of Chemistry and Technology, 153000 Ivanovo, Russia
bN.I. Lobachevsky State University of Nizhni Novgorod, 603950 Nizhny Novgorod, Russia
@Corresponding author E-mail: stuzhin@isuct.ru
DOI: 10.6060/mhc190970s
Macroheterocycles 2020 13(1) 19-22
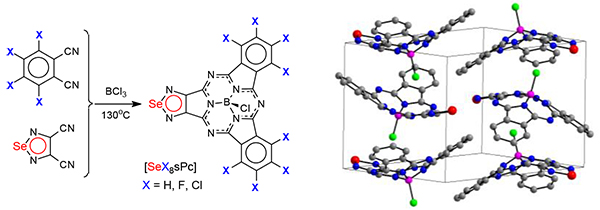
Novel Se-containing heterocyclic subphthalocyanine analogue – (1,2,5-selenadiazolo)dibenzosubporphyrazinatoboron(III) chloride 3a was prepared by template cross-cyclomerization of 1,2,5-selenadiazolo-3,4-dicarbonitrile 1 and phthalonitrile 2a with BCl3 in p-xylene. Similar reactions of 1 and tetrafluoro- or tetrachlorphthalonitriles (2b or 2c) afford octafluorinated and octachlorinated subporphyrazines 3b and 3c. The formation of 3a-c was established by MALDI-TOF mass-spectrometry and the structure of 3a was established by single crystal X-ray diffraction. Influence of halogenation on the spectral properties is discussed.
| Attachment | Size |
|---|---|
| mhc190970s.pdf | 2 MB |
- 1441 reads
- Русский
