Журнал "Макрогетероциклы"
Navigation
News
Impact Factor 2021 = 1.200 has been issued by ISI Web of Knowledge (JCR 2021).
Search
ISSN 1998-9539
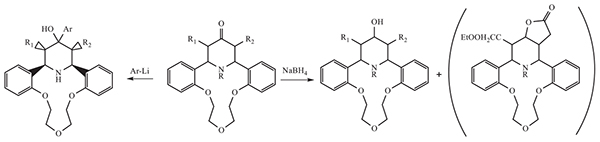
Transformations of (γ-Piperidono)dibenzoaza-14-crown-4 Ethers
Truong H. Hieu,a,e Alexandra I. Komarova,b Alexander N. Levov,b Anatoly T. Soldatenkov,b† Elena I. Polyakova,b Nguyen V. Tuyen,a,d Dang T. T. Anh,a,d Alena N. Kulakova,b Victor N. Khrustalev,b and Le T. Anhc@
aInstitute of Chemistry, Vietnam Academy of Science and Technology (VAST), Hanoi, Vietnam
bDepartment of Inorganic Chemistry, Peoples’ Friendship University of Russia (RUDN University), 117198 Moscow, Russian Federation
cFaculty of Chemistry, VNU University of Science, Vietnam National University, Hanoi, Vietnam
dGraduate University of Science and Technology, VAST, Hanoi, Vietnam
eSaigon Pharmaceutical Science-Technology Centre, University of Medicine and Pharmacy at Ho Chi Minh City, 70000 Ho Chi Minh City, Vietnam
@Corresponding author E-mail: huschemical.lab@gmail.com
DOI: 10.6060/mhc190554a
Macroheterocycles 2019 12(4) 409-414
The transformations of dibenzo[(γ-piperidono]aza-14-crown-4 ethers were implemented with Li-organic compounds and sodium borohydride under reduction condition. The structures of new compounds were confirmed by 1H NMR, IR, MS analysis. X-Ray single-crystal structure study has exactly determined the structure of the representative compound 2g. According to the PASS program, these obtained new azacrown ethers containing γ-piperidol fragment are interested to develop new biologically active agents such as a CYP2H substrate (60–95 %), membrane permeability inhibitor (60–74 %) and spasmolytic agent (60–92 %).
| Attachment | Size |
|---|---|
| mhc190554a.pdf | 1.65 MB |
- 1541 reads
- Русский
