Журнал "Макрогетероциклы"
Navigation
News
Impact Factor 2021 = 1.200 has been issued by ISI Web of Knowledge (JCR 2021).
Search
ISSN 1998-9539
Pyropheophorbide-Fullerene Dyad: Synthesis and Photochemical Properties
Alexander Yu. Rybkin,a@ Alexandra Yu. Belik,a Pavel A. Tarakanov,a,c Kamil R. Taziev,b,a Alexei V. Kozlov,a Nikolay S. Goryachev,a,b Ilya V. Sulimenkov,d Viatcheslav I. Kozlovskiy,d,c Yulia V. Romanenko,e Oscar I. Koifman,e and Alexander I. Kotelnikova,b
aInstitute of Problems of Chemical Physics RAS, 142432 Chernogolovka, Russia
bLomonosov Moscow State University, 119991 Moscow, Russia
cInstitute of Physiologically Active Compounds RAS, 142432 Chernogolovka, Russia
dTalrose Institute for Energy Problems of Chemical Physics (Branch) RAS, 142432 Chernogolovka, Russia
eIvanovo State University of Chemistry and Technology, 153000 Ivanovo, Russia
@Corresponding author e-mail: alryb@icp.ac.ru
DOI: 10.6060/mhc190446r
Macroheterocycles 2019 12(2) 181-186
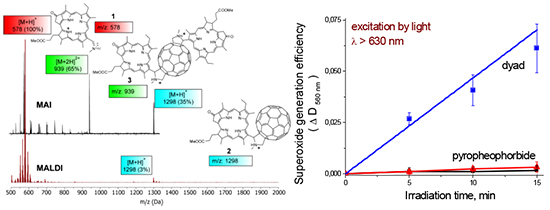
The methylpyropheophorbide-fullerene[60] dyad was synthesized by 1,3-dipolar cycloadditions of the corresponding azomethine ylide to C60 (Prato reaction). Using the mass spectrometric method with soft matrix-activated ionization it was possible to achieve a significant reduction in fragmentation processes by the retro-Diels-Alder reaction, which allows to reliably detect the presence of polyadducts of azomethine ylide cycloadditions to fullerene. The use of gel permeation chromatography under conditions of weakening of the intermolecular π-π interaction between methylpyropheophorbide and fullerene moieties makes it possible to effectively separate mixed products with ~ 1.5 fold difference in molecular weight. It has been shown that the fluorescence of the dyad is quenched more than 5000 times (compared to the native dye). The singlet oxygen quantum yield of the dyad is 360 times less than that for the native methylpyropheophorbide a, however, its efficiency of superoxide generation increases by 18.5 times. The obtained result agrees well with the previously reported mechanism of relaxation of the excited state of the dyad through a charge-separated state, which can lead to the formation of superoxide. The observed effects indicate a change in the mechanism of photodynamic activity from type II (generation of singlet oxygen) for the native dye to type I (generation of superoxide) for the dyad, which shows a promising method of creation of highly efficient photosensitizers based on similar dye-fullerene[60] dyads.
| Attachment | Size |
|---|---|
| mhc190446r.pdf | 1.28 MB |
| mhc190446r_supp.pdf | 1.67 MB |
- 1965 reads
- Русский
