Журнал "Макрогетероциклы"
Navigation
News
Impact Factor 2021 = 1.200 has been issued by ISI Web of Knowledge (JCR 2021).
Search
ISSN 1998-9539
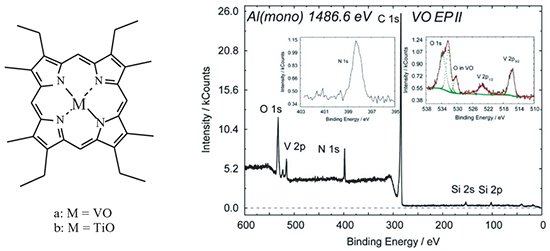
XPS and IR Spectroscopic Studies of Titanyl and Vanadyl Complexes with Etioporphyrin II
Tatyana A. Ageeva,a Denis V. Golubev,b Anastasiya S. Gorshkova,b,c Andrey M. Ionov,d Elena V. Kopylova,b Oskar I. Koifman,a Rais N. Mozhchil,d Ekaterina P. Rozhkova,e Valentina D. Rumyantseva,b,c Aleksander S. Sigov,b and Valeriy V. Fomichevb@
aIvanovo State University of Chemistry and Technology, 153000 Ivanovo, Russia
bMIREA – Russian Technological University, 119571 Moscow, Russia
cKotel’nikov Institute of Radio Engineering and Electronics RAS, 141190 Fryazino, Russia
dInstitute of Solid State Physics RAS, 142432 Chernogolovka, Russia
eLLC NPF Fire Protection Engineering Laboratory, 606016 Dzerzhinsk, Russia
@Corresponding author E-mail: valeryfom@rambler.ru
DOI: 10.6060/mhc190442f
Macroheterocycles 2019 12(2) 148-153
The study is devoted to search for organometallic complexes with considerable dipole moments suitable for the creation of new electret materials. The latter are formed by intercalation of organometallic molecules with a high dipole moment into a polymeric matrix. Titanyl and vanadyl complexes with etioporphyrin II were synthesized. The obtained compounds were identified with the use of electronic spectroscopy, NMR spectroscopy, mass spectrometry and liquid chromatography, and studied by vibrational spectroscopy and X-ray photoelectronic spectroscopy. Quantum chemical calculations for the optimization of the geometry of etioporphyrin II complexes with vanadyl and titanyl cations were performed. Their vibration spectra, dipole moments and distribution of charge and spin densities were calculated. Mulliken population analysis was carried out. It was shown that the “apix” bond between the metal in the vanadyl complex with etioporphyrin II is of more pronounced covalent nature. In contrast, in case of titanyl a structure with some redistribution of electron density onto the oxygen atom is implemented. This increases the polarity of the “apix” Ti–O bond and the dipole moment of the titanyl macrocomplex in general. The calculated dipole moment of the titanyl complex is higher (2.94 D) than that of the vanadyl complex (2.32 D).
| Attachment | Size |
|---|---|
| mhc190442f.pdf | 1.12 MB |
| mhc190442f_supp.pdf | 246.52 KB |
- 1286 reads
- Русский
