Журнал "Макрогетероциклы"
Navigation
News
Impact Factor 2021 = 1.200 has been issued by ISI Web of Knowledge (JCR 2021).
Search
ISSN 1998-9539
Electrodeposition and Characterization of Polyporphyrin Films Based on Mn-Complexes of Amino-substituted Tetraphenylporphyrins
Vladimir I. Parfenyuk,a,b@ Mariya V. Tesakova,a Svetlana A. Chulovskaya,a and Sergey M. Kuzmina,c
aG.A. Krestov Institute of Solution Chemistry of RAS, 153045 Ivanovo, Russia
bIvanovo State University of Chemistry and Technology, 153000 Ivanovo, Russia
cIvanovo State Power Engineering University, 153003 Ivanovo, Russia
@Correspondingauthor E-mail: vip@isc-ras.ru
DOI: 10.6060/mhc190232p
Macroheterocycles 2019 12(2) 154-164
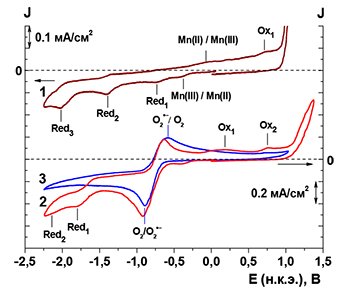
The paper presents the results of the study of electrochemical deposition and physicochemical properties of polyporphyrin films deposited on glassy carbon, platinum, gold and ITO-electrodes from dichloromethane (DCM), ethanol and dimethyl sulfoxide (DMSO). Mn(III) 5,10,15,20-tetrakis(3-aminophenyl)porphyrin and Mn(III) 5,10,15,20-tetrakis(4-aminophenyl)porphyrin chlorides were used as precursors. It was shown that a polyporphyrin film is formed from DMSO in the region of the electroreduction potential of oxygen. The formation of films from DCM and ethanol on the working electrodes in the process of electro-oxidation of the porphyrin monomer is observed in the potential range from 0 to +2 V. The process of electropolymerization has been studied using the quartz crystal microbalance method. The mass growth of the MnClT(3-NH2Ph)P film during deposition from DCM in the potentiostatic mode is 1.9 times greater than in the potentiodynamic mode. The calculated number of electrons participating in the electropolymerization process of MnClT(3-NH2Ph)P in DCM is 5.5. The surface of MnClT(3-NH2Ph)P films obtained from DCM under different deposition modes is rather various. The film obtained at a given potential (+2 V) has a smooth surface with formations of indeterminate shape with a size of 0.5–2 microns. The film obtained in the potentiodynamic mode is visually looser, with a porous surface. The thickness of the poly-MnClT(3-NH2Ph)P film obtained in the potentiodynamic mode is slightly larger than that obtained in the potentiostatic mode (6.81 μm) and is 8.42 μm. The electrochemical properties of MnClT(3-NH2Ph)P and MnClT(4-NH2Ph)P in DMSO solutions and the features of electrochemical deposition of films using superoxide anion radical are studied in detail in this work. In a deaerated solution of porphyrins in DMSO, a quasi-reversible process of changing the oxidation state of the central ion Mn(III)/Mn(II) is observed, which for meta-substituted porphyrin is at more positive potentials than for para-substituted. The potentials of irreversible processes of electroreduction and electro-oxidation of the macrocycle of meta-substituted porphyrin are also more positive than those of para-substituted. On cyclic voltammograms (CV) of porphyrin solutions in DMSO saturated with oxygen there was a difference in the potentials of redox processes associated with porphyrin or oxygen from the potentials of the same processes occurring in pure DMSO without porphyrin (for oxygen) or in deaerated (without oxygen) porphyrin solution (for porphyrin). Вesides, additional channels of oxidation arise. We believe that changes in CV indicate the formation of oxocomplexes in which the metal acquires a stable oxidation state. In deaerated solutions of the studied porphyrins in DMSO, cycling the potential of the working electrode leads to insignificant changes in CV from cycle to cycle. In contrast, in saturated with oxygen solutions, a characteristic change occurs in the CV form from cycle to cycle. The peak of electro-oxidation of a macroheterocycle, observed in the case of the simultaneous presence of porphyrin and dissolved oxygen in DMSO is shifted to the region of positive potentials. The current density of electroreduction near the –2 V potentials decreases markedly. The difference in the change in electrochemical responses appears for potentials of about +1 V: an increase in the current peak occurs at formation of poly-MnClT(4-NH2Ph)P film and the current decreases at formation of Poly-MnClT(3-NH2Ph)P film. The formation of a film associated with the peak of oxygen electroreduction is observed on the Pt, Au, and ITO electrodes. The material of the electrode affects the amplitudes of the currents of the RedOx processes, the magnitude of the mass growth in certain ranges of potentials, and the processes of desorption of the deposited layer. MnClT(3-NH2Ph)P film deposition processes cover wider areas of potentials on ITO than on Pt. At the same time, at the initial stage of film formation, the rate of weight gain on ITO is lower than on Pt. The weight gain in the potential range from +0.5 to +0.8 V (the area of electro-oxidation of porphyrin) is less than on Pt by almost 10 times. At the Au electrode desorption of the coating formed in the region of electroreduction of oxygen is observed starting from the first cycle. With further cycling of the potential, desorption in the potential range from +0.6 to +0.8 V begins to predominate over the film deposition processes, as evidenced by the low value of the film mass growth on the Au electrode. MnClT(4-NH2Ph)P is characterized by a larger film mass increase associated with the potential range of the oxygen electroreduction. This fact correlates with a higher rate of O2•− interaction with para-substituted aminophenyl porphyrins than with meta-substituted ones. Changing the cycling range (from –1.8 to 0 V instead of the range from –1.8 to +0.8 V) leads to a decrease in the mass of film deposited for 10 cycles. At the same time, the decrease in the mass of the film formed is associated not only with the exclusion of film formation processes due to direct oxidation of porphyrin during the first few cycles, but also with a decrease in the mass of the film deposited in the region of oxygen electroreduction potential.
| Attachment | Size |
|---|---|
| mhc190232p.pdf | 1.99 MB |
- 1233 reads
- Русский
