Журнал "Макрогетероциклы"
Журнал является форумом специалистов, изучающих макрогетероциклические соединения
Navigation
News
Impact Factor 2021 = 1.200 has been issued by ISI Web of Knowledge (JCR 2021).
Search
ISSN 1998-9539
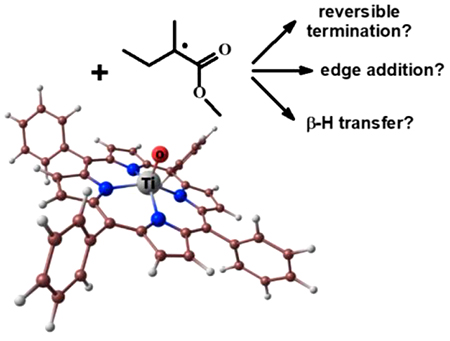
The Reactions of Oxotitanium(IV) Tetraphenylporphyrin with Methyl Methacrylate Radicals: A DFT Study
Anna K. Friesen
Ufa Institute of Chemistry - Subdivision of the Ufa Federal Research Centre of the Russian Academy of Sciences, 450054 Ufa, Russia
E-mail: friesenak@rambler.ru
DOI: 10.6060/mhc181112f
Macroheterocycles 2018 11(4) 371-377
A theoretical analysis of potential reactions between oxotitanium(IV) tetraphenylporphyrin with methyl methacrylate radicals was made. The structures of possible products were calculated and the energies of their formation were estimated. The oxotitanium(IV) tetraphenylporphyrin ((TPP)Ti=O) and the CH3-CH2-C•(CH3)(C(O)OCH3) radical, which simulated a poly(methyl methacrylate) propagating radical, were used as the model particles for quantum-chemical calculations. It has been shown that addition of the radical to the titanium atom does not occur, among other reactions the addition of radical to the O atom (extraligand) is the most likely process. As a result, the metal-centered radical (TPP)Ti•–O–R is formed, which can act as a trap for other methyl methacrylate radicals. Such radical addition occurs on the opposite side from the extraligand. In the (TPP)Ti(OR)(R) product, the radical is added to the titanium atom not by the C atom but by the O atom of the carbonyl group. Furthermore, it has been shown that β-H transfer does not occur with participation of the titanium atom belonging to (TPP)Ti=O, but is principally possible with involvement of the extraligand. The β-H transfer involving the titanium atom in (TPP)Ti•–O–R is energetically unfavorable.
| Attachment | Size |
|---|---|
| mhc181112f.pdf | 1.13 MB |
| mhc181112f_supporting.pdf | 6.75 MB |
- 1295 reads
- Русский
