Serhii V. Vakarov,a Оleg А. Varzatskii,a,b Alexander S. Belov,c Alexander A. Pavlov,c Yan V. Zubavichus,d Anna V. Vologzhanina,c and Yan Z. Voloshinc,e@
Dedicated to Academician Aslan Yu. Tsivadze on the occasion of his 75th Birthday
aVernadskii Institute of General and Inorganic Chemistry NASU, 03080 Kyiv, Ukraine
bSC Princeton Biomolecular Research Labs, 01042 Kyiv, Ukraine
cNesmeyanov Institute of Organoelement Compounds RAS, 119991 Moscow, Russia
dKurchatov Complex for Synchrotron and Neutron Investigations, National Research Centre “Kurchatov Institute”, 123182 Moscow, Russia
eKurnakov Institute of General and Inorganic Chemistry RAS, 119991 Moscow, Russia
DOI: 10.6060/mhc171147v
Macroheterocycles 2017 10(4-5) 552-559
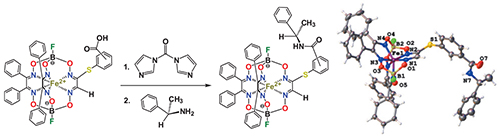
The monoamide-terminated cage complexes FeBd2(X-R(+)-PhCH(CH3)NHOCC6H4S)GmH)(BF)2 (where Bd2– is α-benzyldioxime dianion, Gm is glyoxime residue, X is ortho- or meta-, or para-substituent) were obtained using one-pot two-step synthetic procedure that includes (i) the reaction of its monocarboxyl-terminated clathrochelate precursor with 1,1’-carbonyldiimidazole (CDI), giving the corresponding azaheterocycle-terminated intermediate, and (ii) its cleavage with R(+)-phenylethylamine leading to the target iron(II) clathrochelate with terminal optically active amide group. The complexes obtained were characterized using elemental analysis, MALDI-TOF mass-spectrometry, IR, UV-Vis, 1H and 13C{1H} NMR spectra, and by single crystal X-ray diffraction (for a meta-substituted constitutional isomer). The number, position and integral intensities of the signals in their 1H NMR spectra confirmed the composition of the macrobicyclic molecules. The number of the signals in their 13C NMR spectra suggests the absence of the C2 symmetry axes passing through the middles of the chelate C–C bonds and of the symmetry plane also passing through these points and the encapsulated iron(II) ion as well. As follows from X-ray diffraction data, the encapsulated iron(II) ion in the molecule FeBd2((meta-R(+)-PhCH(CH3)NHOCC6H4S)GmH)(BF)2 is situated in the centre of its FeN6-coordination polyhedron with Fe–N distances falling in the range 1.8904(4)–1.9404(7) Å. This polyhedron possesses the geometry intermediate between a trigonal prism and a trigonal antiprism with the average distortion angle φ of 24.2°; its height h is equal to 2.34 Å and the average bite (chelate) angle α is approximately 78.2°. The terminal PhCH(CH3)NH group of the above clathrochelate molecule is equiprobably disordered over two sites with opposite orientation of its methyl and phenyl substituents; the N–H...F-bonded clathrochelate dimers are formed in its X-rayed crystal..
References:
