Olga A. Omelchuk,a,b@1 Nikita M. Belov,а Vladimir B. Tsvetkov,c,d Alexander M. Korolev,a Lyubov G. Dezhenkova,a Natalia E. Grammatikova,a,e Lyudmila N. Lysenkova,a Olga B. Bekker,f Valery N. Danilenko,f and Andrey E. Shchekotikhina,b@2
aG.F Gause Institute of New Antibiotics, 119021 Moscow, Russian Federation
bD.I. Mendeleev University of Chemical Technology of Russia, 125047 Moscow, Russian Federation
cInstitute for Physical-Chemical Medicine, 119435 Moscow, Russian Federation
dResearch Institute of Influenza, 197376 St. Petersburg, Russian Federation
eI.M. Sechenov First Moscow State Medical University, 119991 Moscow, Russian Federation
fN.I. Vavilov Institute of General Genetics, Russian Academy of Sciences, 119333 Moscow, Russian Federation
DOI: 10.6060/mhc170834o
Macroheterocycles 2018 11(2) 181-192
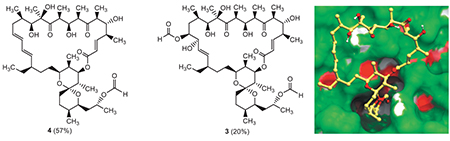
The macrolide antibiotic oligomycin A (1), produced by actinomycetes Streptomyces,[1] is a well-known inhibitor of FOF1 ATP-synthase, which is regarded as a molecular target for new drugs in the treatment of tumors and infections. Oligomycin A (1) exhibits antifungal and cytotoxic activities, but Gram-negative and Gram-positive bacteria are resistant to 1 except actinobacteria.[2] In micromolar concentrations, oligomycin binds to FO c-subunit, blocks proton translocation and disrupts bioenergetic metabolism.[3] However, a clinical application of oligomycin A is limited by high cytotoxicity for mammalian cells. The searches of new derivatives of oligomycin A with more selective pharmacological activity and low toxicity for normal cells are of great interest. New semi-synthetic oligomycins also would be valuable for SAR studies and depicting the mechanism of FOF1 ATP-synthase inhibition. The complicity of oligomycin structure and its lability in basic conditions[4] significantly impede modifications and an applicability of this antibiotic. However, previously we have managed this challenge and developed some modifications of the side chain and chemical transformations of the lactone moiety of 1.[4-9] In this paper, throughout our research we describe synthesis and biological investigation of novel oligomycin A derivatives, namely 16,33-O,O-diformyl-16,17-dihydro-16(S),17(R)-dihydroxyoligomycin A (3) and 33-O-formyloligomycin A (4). First, we have studied Prilezhaev epoxidation of double bonds in core structure of oligomycin A. It was found that treatment 1 with m-CPBA at -17 oC in dichloromethane led to 16,17-epoxyoligomycin (2). Unfortunately, all attempts for isolation of product 2 were failed due to its instability on silica gel, and, consequently, we were unable to determine the structure of 2 by direct physicochemical and spectral methods. The presence of epoxide at C16-C19 positions was confirmed by tandem mass spectrometry, but its exact localization was still elusive. We assumed that it might be at C16-C17 positions, because C18-C19 double bond is hindered by ethyl side chain at C20. In order to obtain a stable oligomycin A derivative we performed an epoxide ring-opening reaction by the treatment of the crude epoxyoligomycin 2 with formic acid. This acid-catalyzed opening of the epoxide accompanied with acylation of 33-OH group and led to16,33-O,O-diformyl-16,17-dihydro-16(S),17(R)-dihydroxyoligomycin A (3). The structure of 3 was confirmed by NMR spectroscopy and high resolution mass spectrometry. Configurations at C16 and C17 positions were determined by detecting correlations in 1H-1H ROESY spectrum. Obtained results allowed to confirm an assumption about localization of the epoxide ring and establish the structure of 2 as (R,R)-16,17-epoxyoligomycin A, since inversion of configuration has taken place at the attacked carbon atom.[23] It is known that O-acyl derivatives of pharmacologically active agents are widely used as prodrugs.[24] Acylation of 2-hydroxypropyl side chain in 2 prompts us to examine the reaction of oligomycin A (1) with formic acid. Thus, stirring the solution of 1 in HCOOH (98 %) for 2 h at room temperature afforded 33-O-formyloligomycin A (4) in a good yield. The structure of 4 was confirmed by NMR-spectroscopy and high resolution mass spectrometry. Also, biological data of new derivatives were evaluated. The modification of C16-C17 positions of the macrocycle as well as acylation of C33 hydroxyl group led to the decreasing of activity against S. fradiae, Candida spp. and filamentous fungi. Obtained results were in agreement with docking studies. A simulation of an interaction of 1, 3 and 4 with the FO subunit of the ATP-synthase (PDB: 4f4s) revealed that these modifications led to a significant change in the solvation energy and an increase in the conformational capacity of the ligands during the binding with the target. This resulted in decrease of the binding affinity for derivatives 2, 3. However, 33-O-formyloligomycin A (4) showed similar antiproliferative activity against tumor cell lines (HCT-116 colon carcinoma, К562 myeloid leukemia cell lines and MDR K562/4 subline) as for 1, butless cytotoxic for non-malignant human cells.
References: