I. V. Smirnov,a,b,c@ E. S. Stepanova,a,b M. Yu. Tyupina,a N. M. Ivenskaya,b S. R. Zaripov,d S. R. Kleshnina,b,d S. E. Solovieva,e and I. S. Antipind,e
aKhlopin Radium Institute, 194021 St. Petersburg, Russian Federation
bOzersk Institute of Technology, 456783 Ozersk, Russian Federation
cSaint Petersburg State University, 199034 St. Petersburg, Russian Federation
dKazan (Volga Region) Federal University, 420008 Kazan, Russian Federation
eA.E. Arbuzov Institute of Organic and Physical Chemistry, 420088 Kazan, Russian Federation
DOI: 10.6060/mhc161070s
Macroheterocycles2017 10(2) 196-202
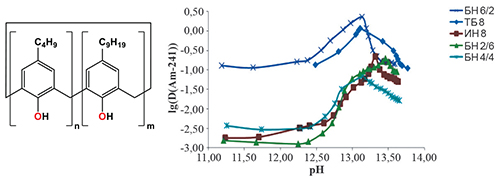
The recovery of long-lived radionuclides from liquid radioactive wastes with subsequent immobilization and disposal significantly reduces radiological risks in the nuclear industry. At the Savannah River Site (USA), the functionalized calixarene bis(dimethyloctyl-oxy)calix[4]arenebenzocrown-6 (MaxCalix) was successfully used as selective extractant to separate cesium-137 from alkaline radioactive wastes. At the Mayak PA (Russia), the alkaline radioactive wastes include actinides, essentially americium, in addition to cesium-137, which also should be recovered. To separate cesium and americium from alkaline solutions, the simple tert-butylcalix[8]arene could be used, if it was not so poorly soluble in organic solvents. The aim of this study was to synthesize mixed p-alkylcalix[8]arenes with tert-butyl and isononyl groups taken at various ratios and to examine their applicability to extract cesium and americium from alkaline solutions. Starting from commercially available p-isononylphenol we have synthesized and characterized three mixed tert-butyl-isononylcalix[8]arenes with the tert-butyl to isononyl group ratio taken as 6:2, 4:4, and 2:6. The solubility of these mixed tert-butyl-isononylcalix[8]arenes in tetrachloroethylene ranges from 0.01 to 0.08 М, which is 3 to 8 times higher than that of p-tert-butylcalix[8]arene. The extraction of 137Cs and 241Am from alkaline-carbonate solutions with mixed p-alkylcalix[8]arenes in tetrachloroethylene was studied. For all the extractants, the maximal extraction of cesium and americium was observed in the pH range 13–14. Americium is extracted at lower pH since, unlike cesium, it forms very strong hydroxo complexes at high pH values. The composition of the solvates formed by the calixarenes with the radionuclides in the organic phase was determined using the equilibrium shift method (for trace amounts of the radionuclides) and the saturation method (extraction isotherm). The results obtained by the equilibrium shift method revealed dominant formation of solvates, in which the p-alkylcalix[8]arene binds 1–2 cations of cesium or americium. The saturation method suggests formation of solvates containing up to four cesium cations per an p-alkylcalix[8]arene molecule. Step-by-step substitution of the tert-butyl groups in p-alkylcalix[8]arene for the isononyl groups leads to decreasing extraction of both cesium and americium. For cesium, a small monotonic decrease in the extraction power of p-alkylcalix[8]arenes was observed: the difference in the distribution ratios between the tert-butyl and isononyl derivatives of calix[8]arene was less than two. The highest extractability of americium was observed with the mixed calix[8]arene containing two isononyl groups, and the difference in the distribution ratios was above 20. Interesting results were obtained when comparing the extraction of americium with freshly prepared and aged tetrachloroethylene solutions of the mixed calix[8]arene containing two isononyl groups. The americium distribution ratios obtained with the freshly prepared extractant was found to be lower by a factor of 4-5 as compared to the extractant aged for 15 h at +5 °C. The data obtained by the dynamic light scattering method indicate that, in freshly prepared solutions, the calix[8]arene occurs in a monomeric form, which transforms with time into large aggregates with a diameter of about 8 nm to form inverse micelles.
References:
