Журнал "Макрогетероциклы"
Navigation
News
Impact Factor 2021 = 1.200 has been issued by ISI Web of Knowledge (JCR 2021).
Search
ISSN 1998-9539
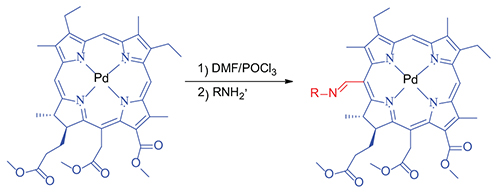
Palladium Complexes of Azomethine Derivatives of Porphyrins as Potential Photosensitizers
V. S. Tyurin,a D. R. Erzina,a I. A. Zamilatskov,a@ A. Yu. Chernyadyev,a G. V. Ponomarev,b D. V. Yashunskiy,b A. V. Maksimova,a A. A. Krasnovskiy,c and A. Yu. Tsivadzea
Macroheterocycles 2016 9(4) 462
Various isomers of azomethine derivatives of mesoporphyrin IX were first obtained in the form of palladium complexes by the interaction of the so-called “phosphorus complex”, produced in situ in the Vilsmeier-Haack reaction of allylamine and methylamine. The compounds were identified and characterized using the methods of NMR 1D and 2D NOESY, and electronic spectroscopy, and mass spectrometry. Photophysical studies of some palladium complexes of meso-(methylimino) and allylimino-derivatives of coproporphyrin I, mesoporphyrin IX and mesochlorin e6 were carried out. Absorption and photoluminescence (phosphorescence) spectra were studied, and lifetimes of phosphorescence were determined, which were high enough, being several tens of microseconds which corresponds to the previously studied analogous compounds. Peripheral substituents had insignificant effect on the position of the absorption bands and the lifetimes of phosphorescence, therefore, subsequent modification of the azomethine fragment should not significantly change the photophysical properties of the resulting target photosensitizer. The quantum yields of generation of singlet oxygen for the part of the investigated compounds were determined to be quantitative, what implies quantum yield of the triplet excited state of them to be equal to one. The results demonstrate the using prospects of the studied compounds as precursors for subsequent functionalization to obtain potential photosensitizers for photo-oxidation processes with molecular oxygen, in particular, for photodynamic therapy of cancer through the transformation of the azomethine group with the aim of obtaining conjugates of these porphyrins with functional fragments, facilitating efficient transport and selective penetration of photosensitizers in tumor tissue. A preliminary assessment of the photophysical properties of the substances studied allows to position these compounds as precursors for the synthesis of the third generation photosensitizers, which are more suitable for the treatment of superficial cancers due to the shallow penetration of light at the absorption wavelength of these compounds.
| Attachment | Size |
|---|---|
| mhc151199z.pdf | 615.38 KB |
| mhc151199z-Supplementary.pdf | 2 MB |
- 2010 reads
- Русский
