Журнал "Макрогетероциклы"
Navigation
News
Impact Factor 2021 = 1.200 has been issued by ISI Web of Knowledge (JCR 2021).
Search
ISSN 1998-9539
New Amphiphilic Bowl-Shaped Receptors on the Basis of Calix[4] arenes in Cone Conformation: Synthesis, Self-Aggregation and Eosin Y Dye Binding
Vladimir A. Burilov,a@ Guzalia A. Fatikhova,a Diana A. Mironova,a Svetlana E. Solovieva,a,b and Igor S. Antipina,b
aKazan Federal University, 420008 Kazan, Russian Federation
bA.E. Arbuzov Institute of Organic and Physical Chemistry, 420088 Kazan, Russian Federation
@Corresponding author E-mail: ultrav@bk.ru
DOI: 10.6060/mhc151087b
Macroheterocycles 2015 8(4) 409-414
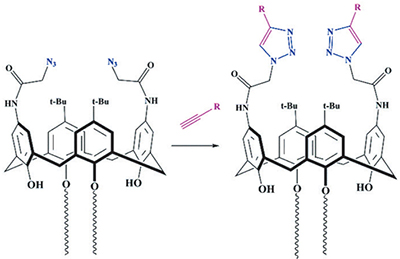
The first calix[4]arene derivative in cone conformation containing two azidoacetamide groups on the upper rim and two alkyl substituents on the lower rim of the macrocycle was synthesized. It has been shown that this compound is a convenient precursor for the synthesis of broad series of amphiphilic receptors on the calixarene platform in cone stereoisomeric forms using the copper-catalyzed 1,3-dipolar cycloaddition (CuAAC) to terminal alkynes. The formation of 1,2,3-triazoles have been established by one- and two-dimensional NMR spectroscopy. Water soluble bis-ammonium derivative possesses the receptor and surfactant properties. The formation of 2:1 complex (eosin:calixarene) with a negatively charged fluorescent Eosin Y dye was accompanied by a significant quenching of dye fluorescence. This “dark” complex is perspective for the design of the fluorescent sensors for anionic organic substrates, based on the competitive displacement of the dye molecules in the calixarene cavity. Above the critical aggregation concentration (3.3∙10-4 M) two types of nanoaggregates with diameter of 27 and 139 nm are observed, where in the complex stoichiometry remains unchanged.
| Attachment | Size |
|---|---|
| mhc151087b.pdf | 878.11 KB |
- 2113 reads
- Русский
