Журнал "Макрогетероциклы"
Navigation
News
Impact Factor 2021 = 1.200 has been issued by ISI Web of Knowledge (JCR 2021).
Search
ISSN 1998-9539
New P,N-Containing Cyclophanes with Exocyclic Pyridyl- Containing Substituents on Phosphorus Atoms
Yulia A. Nikolaeva, Anna S. Balueva,@ Elvira I. Musina,Andrey A. Karasik, and Oleg G. Sinyashin
A.E. Arbuzov Institute of Organic and Physical Chemistry of Kazan Scientific Center, Russian Academy of Sciences, 420088 Kazan, Russian Federation
@Corresponding author E-mail: balueva62@mail.ru
DOI: 10.6060/mhc150976b
Macroheterocycles 2015 8(4) 402-408
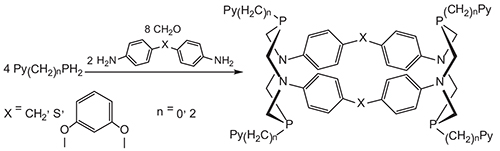
The synthesis of 28- and 36-membered P,N-containing cyclophanes with two 1,5-diaza-3,7-diphosphacyclooctane rings in the macrocyclic frameworks and exocyclic pyridine-2-yl or 2-(pyridine-2-yl)ethyl substituents on all phosphorus atoms is described. These cyclophanes have been obtained by Mannich-like condensations in three components systems: pyridyl-containing primary phosphines–formaldehyde–primary diamines with spatially divided amine groups (bis(4-aminophenyl)methane, bis(4-aminophenyl)sulfide or 1,3-bis(4’-aminophenoxy)benzene), and the selectivity of the macrocyclization depends on the lengths the diamine spacers unlike previously described analogous condensations with the participation of arylphosphines. The condensations of pyridyl-containing phosphines with formaldehyde and diamines containing relatively short spacers formed by two phenylene groups bonded with one-atom bridges proceed as covalent self-assembly and lead to the predominant formation of 28-membered cyclophanes which have been isolated in moderate yields (17–21 %) whereas the condensations with the participation of 1,3-bis(4’-aminophenoxy)benzene containing three phenylene groups in the spacer are much less selective, and 36-membered cyclophanes have been obtained in low yields (7–9 %). It shows the strong influence of the starting phosphine nature on the selectivity of the Mannich-like condensation reactions. The treatment of 28-membered P-pyridylethyl substituted cyclophanes with hydrochloric acid in chloroform leads to the corresponding monohydrochlorides as a result of the protonation of pyridyl nitrogen atom, and in the solutions the fast migration of the proton between all pyridyl nitrogen centers is observed.
| Attachment | Size |
|---|---|
| mhc150976b.pdf | 502.93 KB |
- 1724 reads
- Русский
