Журнал "Макрогетероциклы"
Журнал является форумом специалистов, изучающих макрогетероциклические соединения
Navigation
News
Impact Factor 2021 = 1.200 has been issued by ISI Web of Knowledge (JCR 2021).
Search
ISSN 1998-9539
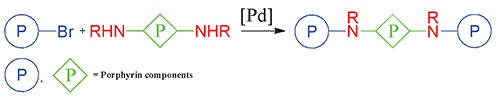
Synthesis of New Porphyrin Trimers via Buchwald-Hartwig Amination Reaction
Vladimir S. Tyurin,a Elena A. Mikhalitsyna,a Alexandr S. Semeikin,c and Irina P. Beletskayaa,b@
aA.N. Frumkin Institute of Physical Chemistry and Electrochemistry, Russian Academy of Sciences, 119071 Moscow, Russian Federation
bM.V. Lomonosov Moscow State University, Chemistry Department, 119992 Moscow, Russian Federation
cIvanovo State University of Chemistry and Technology, 153000 Ivanovo, Russian Federation
@Corresponding author E-mail: beletska@org.chem.msu.ru
DOI: 10.6060/mhc150769b
Macroheterocycles 2015 8(4) 358-365
Two types of the novel porphyrin trimers linked by amines were synthesized by the Buchwald-Hartwig amination reaction of zinc complex of 5-(4-bromophenyl)-b-octaalkylporphyrin with zinc 5,15-di(4-aminophenyl)-10,20-dimesitylporphyrin, and zinc 5,15-diaryl-b-octaalkylporphyrins, where aryl groups were functionalized with piperazine or diaza-18-crown-6 ether. One type of trimers was bridged by one nitrogen atom of diphenylamine and another type of trimers was linked through two different nitrogene atoms of the cyclic diamines. Each type of the trimers has required specific catalyst composition varying in the ligands for catalytic palladium complex: BINAP and DavePhos correspondingly. The highest 75 % yield was achieved for the piperazine linked trimer. UV-Vis spectroscopic investigations of the synthesized compounds were carried out and showed that the observed spectra of the trimers consist of the spectra sum of their porphyrin components. Thus the trimers have shown negligible interchromophore interaction in both ground and excited states. The geometry of the trimer bridged by diphenylamine was optimized by molecular mechanics MM+ method showing angled structure with the distance between centers of the macrocycles of 16.86 Å. The interaction of the chromophores should be weak on such a distance, and there is no conjugation of electron systems of chromophores due to the orthogonal orientation of the bridging benzene rings relative to the tetrapyrrole plane. These results of the geometry calculations well correspond to the spectroscopic observations.
| Attachment | Size |
|---|---|
| mhc150769b.pdf | 738.11 KB |
| MHC150769b-Supplementary.pdf | 2.49 MB |
- 1861 reads
- Русский
