Журнал "Макрогетероциклы"
Navigation
News
Impact Factor 2021 = 1.200 has been issued by ISI Web of Knowledge (JCR 2021).
Search
ISSN 1998-9539
X-Ray Single-Crystal Structures and NMR Characterization of Three Vinyl Substituted Methylpyropheophorbide a Derivatives
Ivan S. Lonin,a@ Evgeny S. Belyaev,a Viktor A. Tafeenko,b Vladimir V. Chernyshev,a,b Elena V. Savinkina,c Gelii V. Ponomarev,d Oskar I. Koifman,eand Aslan Yu. Tsivadzea
aA.N. Frumkin Institute of Physical Chemistry and Electrochemistry, Russian Academy of Science, 199071 Moscow, Russia
bDepartment of Chemistry, M.V. Lomonosov Moscow State University, 119991 Moscow, Russia
cMoscow State University of Fine Chemical Technologies,119571 Moscow, Russia
dOrekhovich Institute of Biomedical Chemistry, Russian Academy of Medical Sciences,119121 Moscow, Russia
eResearch Institute of Macroheterocycles, Ivanovo State University of Chemistry and Technology, 153000 Ivanovo, Russia
@Corresponding author E-mail: loninis@gmail.com
DOI: 10.6060/mhc150457l
Macroheterocycles 2015 8(4) 366-370
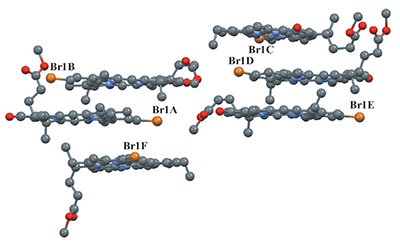
Three recently synthesized tetrapyrrole compounds: (E)-32-bromopyropheophorbide a methyl ester (1) and its derivatives with p-conjugated acrylate (2) and phenyl (3) substituents are structurally characterized using X-ray diffraction along with 1D and 2D NMR spectroscopy. All compounds are crystallized in chiral space groups. The planar molecules form stacks extended in one direction. The 1H and 13C signals were completely assigned using 2D {1H,1H} correlation spectroscopy (COSY), nuclear Overhauser effect spectroscopy (NOESY), {1H,13C} heteronuclear single quantum coherence (HSQC), and heteronuclear multiple bond correlation (HMBC) experiments. The details of structure elucidation and signal assignment process are given using compound 2 as an example.
| Attachment | Size |
|---|---|
| mhc150457l.pdf | 975.14 KB |
| MHC150457-Supplementary.pdf | 296.59 KB |
- 1697 reads
- Русский
