Журнал "Макрогетероциклы"
Navigation
News
Impact Factor 2021 = 1.200 has been issued by ISI Web of Knowledge (JCR 2021).
Search
ISSN 1998-9539
Theoretical and Experimental Study of Inclusion Complex Formation of β-Cyclodextrin with Some 1,4-Diazepine Derivatives
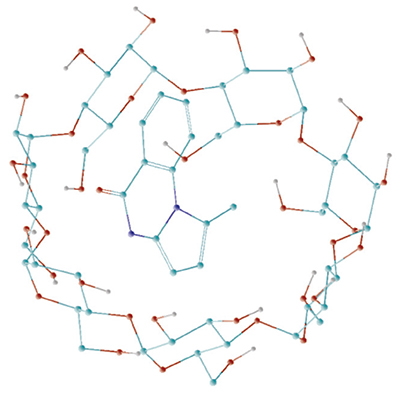
Benzodiazepines belong to a group of heterocyclic compounds that are widely used as pharmaceuticals. They have a wide spectrum of pharmacological effects including anxiolytic, sedative, hypnotic, anticonvulsant, muscle relaxant and amnesic activity. Ten novel fused 1,4-diazepines were examined using the PASS Online service in order to evaluate their anticipated biological activity. Virtual screening check list was composed of 6 essential psychotropic activities such as antipsychotic (neuroleptic), antidepressant, tranquilizing (anxiolytic), muscle relaxant, nootropic and anticonvulsant. Screening results showed that 1-methyl-4,5-dihydro-6H-pyrrolo[1,2-a][1,4]benzodiazepin-6-one the most likely would have nootropic effect while the N-(tert-butyl)-2-(1-methyl-6-oxo-4H-pyrrolo[1,2-a][1,4]benzodiazepin-5(6H)-yl)acetamide has been recognized as neuroleptic and 8,9-dimethoxy-1-methyl-4,5-dihydro-6Н-pyrrolo[1,2-a][1,4]benzodiazepine-6-tione was expected to have an anticonvulsant effect. Drug delivery systems, especially those based on cyclodextrin inclusion complexes are the most promising ways towards enhanced pharmaceutical forms. Cyclodextrins are cyclic oligosaccharides composed of six to eight dextrose units joined through one to four α-D bonds. They have hydrophilic outer surface and a hydrophobic axial open cavity, which is capable to encapsulate a great variety of organic and inorganic compounds through the formation of inclusion complexes. This property of cyclodextrins has been used in the pharmaceutical industry to improve the physical and chemical properties (water solubility, stability, dissolution rate) of various drug molecules. The complexes of β-cyclodextrin with non-steroidal anti-inflammatory drugs (e.g., paracetamol, ibuprofen, ketoprofen, flufenamic and mefenamic acids, etc.), steroids, prostaglandins and prostacyclins, barbiturates, sulfonamides, cardiac glycosides and many other drugs are known. Moreover, cyclodextrins are nontoxic and inexpensive substances. The inclusion complex of 1-methyl-4,5-dihydro-6H-pyrrolo[1,2-a][1,4]benzodiazepin-6-one with β-cyclodextrin was synthesized by kneading method. The obtained complex compound was characterized by solid state NMR, IR spectroscopy and thermal analysis. The formation of inclusion complex leads to changes in the IR spectrum. The ν(OH) band shifts from 3257 cm-1 for β-cyclodextrin to 3278 cm-1 for complex compound which indicates that hydroxyl groups of β-cyclodextrin participate in hydrogen bonding. Similar changes exhibit stretching and bending vibration bands of the amino group but the effect is less than for OH. Thus, δ(N-H) frequency changed from 1624 сm-1 in the ligand up to 1629 сm-1 in the complex compound. The frequency of characteristic stretching vibration of carboxyl group ν(С=О) is decreased from 1657 сm-1 to 1648 сm-1 which could also be considered as a proof that the formation of inclusion complex takes place. Such a minor changes are common for the non-covalent nature of the interaction in the inclusion complexes. The results of thermal analysis showed that the formation of inclusion complex leads to decreasing of thermal stability both the β-cyclodextrin and the ligand. The obtained inclusion complex was studied by solid state NMR. The changes of chemical shifts of the β-cyclodextrin signals caused by the complexation are -0.9 ppm for the C2, C3 and C5 atoms and 0.512 ppm for C4. The main change in the spectrum of complex is that the signals of the individual conformations visible in pure β-CD as multiplets are converged to broad single peaks in complex. In the “guest” molecule the atoms closest to the β-cyclodextrin ring exhibit the largest shifts. This is consistent with the results of quantum chemical calculations. To find the most probable geometry of inclusion complex, molecular docking study was carried out using the AutoDock program. As a result, a number of the most preferred conformations were obtained. The geometry of the obtained conformations was optimized using the semi-empirical method AM1. Based on the docking data, the geometry of the most probable structure of the inclusion complex compound was suggested.
| Attachment | Size |
|---|---|
| mhc191281v.pdf | 1.66 MB |
- 1277 reads
- Русский