Журнал "Макрогетероциклы"
Журнал является форумом специалистов, изучающих макрогетероциклические соединения
Navigation
News
Impact Factor 2021 = 1.200 has been issued by ISI Web of Knowledge (JCR 2021).
Search
ISSN 1998-9539
Synthesis of the Novel Cationic Chlorin Derivatives with a Phytol Fragment on the Periphery of the Macrocycle and Their Aggregation State in Aqueous Surfactant Solutions
Margaret A. Gradova,a@ Tamara G. Movchan,b Irina S. Khudyaeva,c Andrey Yu. Chernyad’ev,b Elena V. Plotnikova,b Anton V. Lobanov,a,d and Dmitry V. Belykhc
aN.N. Semenov Federal Research Centre of Chemical Physics of the Russian Academy of Sciences, 119991 Moscow, Russia
bA.N. Frumkin Institute of Physical Chemistry and Electrochemistry of the Russian Academy of Sciences, 119071 Moscow, Russia
cInstitute of Chemistry of the Federal Research Centre “Komi Scientific Centre of the Ural Branch of the Russian Academy of Sciences”, 167000 Syktyvkar, Russia
dMoscow State Pedagogical University, Institute of Biology and Chemistry, 129164 Moscow, Russia
@Corresponding author E-mail: m.a.gradova@gmail.com
DOI: 10.6060/mhc191072g
Macroheterocycles 2020 13(1) 23-32
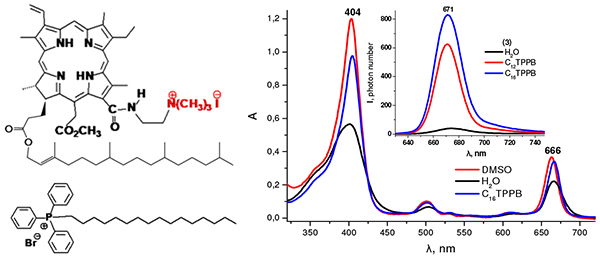
Novel cationic chlorin e6 derivatives with a phytol fragment on the periphery of the macrocycle are synthesized from pheophytin a and can be considered as potential photosensitizers for antimicrobial photodynamic therapy. The presence of positively charged aminomethyl groups in the macrocycle enhances both the hydrophylicity and bioavailability of the compounds studied, as well as provides their interaction with the cell wall of gram-negative bacteria and mitochondrial membranes. The phytol fragment provides an affinity of the photosensitizer towards the cell membrane, but increases its hydrophobicity, and hence, the tendency to form aggregates in polar aqueous media. In this connection, it is necessary to perform solubilization studies of the potential cationic chlorin-based photosensitizers in aqueous media using different solubilizing agents, such as surfactants. In this work the aggregation behavior and photophysical properties of mono- and dicationic chlorin derivatives in water-rich organic media and aqueous microheterogeneous systems were studied by means of absorption and luminescence spectroscopy. The solubilization studies were performed in the micellar solutions of the conventional surfactants: anionic SDS, cationic CTAB and non-ionic TX-100, as well as in the presence of the novel cationic surfactants – dodecyl, tetradecyl and hexadecyl triphenylphosphonium bromides. These surfactants possess their own antimicrobial activity and are more biocompatible compared to alkyl trimethylammonium salts, so they are considered as the prospective drug delivery systems. It was found that in H2O-DMSO mixtures with a high water content cationic chlorin derivatives exist predominantly in an aggregated form, while in pure DMSO they are in a monomolecular fluorescent form. In micellar solutions of different surfactants the monomer to aggregate ratio of cationic chlorins varies depending on the nature of the surfactant and the structure of the chlorin molecule. For both chlorins the highest solubilization efficiency was achieved in micellar solution of TX-100 with the monomer to aggregate ratio higher in the case of a more hydrophilic dicationic derivative. In micellar solutions of alkyl triphenylphosphonium bromides it is possible to achieve stabilization of both cationic chlorins in a monomolecular form at the 2.5 µM chlorin concentration, and the solubilization efficiency does not depend on the number of the alkyl chain length of the surfactant molecule. Moreover, the solubilization effect is observed even at the submicellar surfactant concentration due to the decrease of the cmc value in the presence of amphiphilic cationic chlorin molecules resulting in the premicellar chlorin-surfactant aggregate formation. Chlorin binding to the micelles of alkyl triphenylphosphonium bromides enhances their bioavailability and provides the possibility to design novel supramolecular compositions for antimicrobial photodynamic therapy. Low cmc values of the alkyl triphenylphosphonium salts compared to those of the classical cationic surfactants allow to reduce the toxicity of the potential drug formulations due to the decrease of the surfactant concentration required for the effective stabilization of the monomolecular form of the photosensitizer. Moreover, considering the intrinsic antimicrobial activity of alkyl triphenylphosphonium surfactants, a synergistic interaction between the surfactant and the photosensitizer is expected to be observed, resulting in the presence of both dark and photoinduced cytotoxicity of the potential drug formulations.
| Attachment | Size |
|---|---|
| mhc191072g.pdf | 783.85 KB |
- 1628 reads
- Русский