Журнал "Макрогетероциклы"
Navigation
News
Impact Factor 2021 = 1.200 has been issued by ISI Web of Knowledge (JCR 2021).
Search
ISSN 1998-9539
Self-Organization Features of Tetraphenylporphyrins according to Quantum Chemical Calculations
М. А. Elistratova,a@ I. B. Zakharova,b and О. Е. Kvyatkovskiia
aIoffe Institute, Russian Academy of Sciences, 194021 St. Petersburg, Russia
bPeter the Great St. Petersburg Polytechnic University, 195251 St. Petersburg, Russia
@Corresponding author E-mail: marina.elistratova@mail.ioffe.ru
DOI: 10.6060/mhc190552e
Macroheterocycles 2019 12(4) 370-374
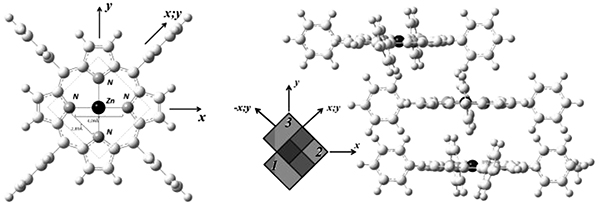
The optimal geometry of molecules of tetraphenylporphyrin (H2TPP), its zinc complex (ZnTPP) and their dimers and trimers, as well as the HOMO-LUMO energy gap and the binding energies of the complexes, and some other parameters were calculated by quantum chemical method. The calculations of the electronic structure of (MeTPP)n complexes were carried out in the framework of the density functional theory with the B3LYP hybrid functional using MOLCAO SCF with atomic sets of basic Gaussian functions 6-311G (2df, 2pd) with polarizing d and f functions. The difference in energy gain during the formation of dimers and trimers gave an explanation of the self-organization features of different types of porphyrins. The connection between configurations of the basic states of trimers and self-organization properties of selected materials is presented. An explanation of the relationship between the type of porphyrin and its self-organization properties is given. The ground states of the ZnTPP and H2TPP trimers have different geometry configuration – “Zig-zag” for ZnTPP and “Stairs” for H2TPP. Due to this fact these materials have different ability to form linear self-organized structures. The energy gain during the formation of the ZnTPP trimer with the optimal configuration is 13.23 kcal/mol, and for H2TPP is 7.79 kcal/mol. It was concluded that ZnTPP is not prone to self-organization into linear structures under normal crystallization conditions. Whereas the ground state of (ZnTPP)3 has a “Zig-zag” geometry, it tends to form planar structures. The large difference between ground and minor states makes it difficult to control self-organization and the growth of nanostructures. The ground state in the geometry of the “Stairs” and the small energy difference between other (H2TPP)3 configurations, on the contrary, allows growing various modifications of structures, including nanowires, with simple technological changes.
| Attachment | Size |
|---|---|
| mhc190552e.pdf | 783.88 KB |
- 1552 reads
- Русский
