Журнал "Макрогетероциклы"
Navigation
News
Impact Factor 2021 = 1.200 has been issued by ISI Web of Knowledge (JCR 2021).
Search
ISSN 1998-9539
Hemihexaphyrazine and Thiadiazole Annulated Hemihexaphyrazine: A Theoretical Insight into Aromaticity and Energetics of Hydrogen Bonding
Arseniy A. Otlyotov,@1 Vladimir V. Veretennikov, Anton P. Merlyan, Evgeny N. Ivanov, Yana E. Filippova, Yuriy A. Zhabanov,@2 and Mikhail K. Islyaikin@3
Institute of Macroheterocycles, Ivanovo State University of Chemistry and Technology (ISUCT), 153000 Ivanovo, Russian Federation
@1Corresponding author E-mail: arseney_otlyotov@mail.ru
@2Corresponding author E-mail: zhabanov@gmail.com
@3Corresponding author E-mail: islyaikin@isuct.ru
DOI: 10.6060/mhc190443o
Macroheterocycles 2019 12(2) 209-214
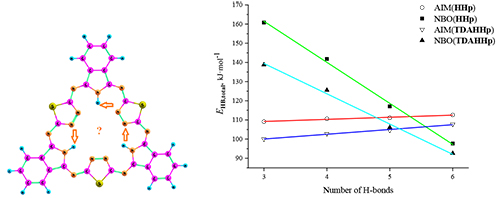
Seventeen tautomeric structures of hemihexaphyrazine were considered using DFT in B3LYP/pcseg-2 approximation. The tautomer of D3h symmetry was found to be the most energetically favorable, and transformations into other tautomers are practically forbidden by high energy barriers (more than 50 kJ·mol-1). The energetics of hydrogen bonding was estimated by NBO and QTAIM calculations and the preferable D3h tautomer turned out to possess the lowest total hydrogen bond stabilization energy, according to the results of the NBO-analysis. Aromaticity of the D3h and C3h tautomers of hemihexaphyrazine and its thiadiazole annulated analogue was described with use of three popular descriptors: NICS, HOMA and FLU. Tautomeric preference and structural features of the both molecules were found to be very similar.
| Attachment | Size |
|---|---|
| mhc190443o.pdf | 1.32 MB |
| mhc190443o_supp.pdf | 1.68 MB |
- 1120 reads
- Русский
