Журнал "Макрогетероциклы"
Журнал является форумом специалистов, изучающих макрогетероциклические соединения
Navigation
News
Impact Factor 2021 = 1.200 has been issued by ISI Web of Knowledge (JCR 2021).
Search
ISSN 1998-9539
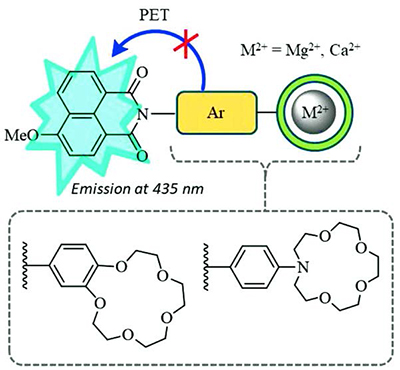
Crown-Containing 4-Methoxy-1,8-naphthalimide Derivatives as a Basis for the Construction of Fluorescent PET Chemosensors for Metal Cations
Pavel A. Panchenko,a,b@ Nikolai V. Leichu,b Yuri V. Fedorov,a and Olga A. Fedorovaa,b
aA.N. Nesmeyanov Institute of Organoelement Compounds of Russian Academy of Sciences, 119991 Moscow, Russia
bD.I. Mendeleev University of Chemical Technology of Russia, 125047 Moscow, Russia
@Corresponding author E-mail: pavel@ineos.ac.ru
DOI: 10.6060/mhc190339p
Macroheterocycles 2019 12(3) 319-323
Two novel 4-methoxy-1,8-naphthalimides bearing benzo-15-crown-5 and N-phenylaza-15-crown-5 ether receptor fragments as N-aryl substituents at imide nitrogen have been synthesized by the nucleophilic substitution of the C-4 nitro groups in the corresponding 4-nitro derivatives. The prepared compounds exhibit long wavelength absorption bands at around 360 nm related to the charge transfer transition in the naphthalimide core and a weak fluorescence at 435 nm. The low values of the fluorescence quantum yields can be explained by the occurrence of the photoinduced electron transfer (PET) process between the donor N-aryl receptor and the acceptor naphthalimide chromophore, which is confirmed by the frontier molecular orbitals calculation by the PM6 method. According to the calculation results, the energy level of the N-aryl local HOMO in both ligands is higher than that of the singly occupied HOMO localized over the excited naphthalimide fragment, thus affording a high driving force for PET. Binding of earth-alkali metal cations (Mg2+, Ca2+) in acetonitrile solution caused the fluorescence intensity enhancement by two orders of magnitude, whereas the changes in the UV/Vis absorption spectra of the studied compounds were negligible. The observed spectral behavior is consistent with the formation of complexes with 1:1 metal to ligand ratio where the PET is hampered. Using the fluorometric titration data, stability constants of complexes and their emission quantum yields have been estimated. Coordination of metal cations with the crown ether moieties was also traced by the 1H NMR spectroscopy. Thus, the presented results have shown that crown-containing 4-methoxy-1,8-naphthalimides could be used as a basis for the construction of fluorescent chemosensors for metal cations with PET mechanism of optical response.
| Attachment | Size |
|---|---|
| mhc190339p.pdf | 1.13 MB |
| mhc190339p_supp.pdf | 7.06 MB |
- 1493 reads
- Русский
