Журнал "Макрогетероциклы"
Журнал является форумом специалистов, изучающих макрогетероциклические соединения
Navigation
News
Impact Factor 2021 = 1.200 has been issued by ISI Web of Knowledge (JCR 2021).
Search
ISSN 1998-9539
Features of Inverted Tetrasulfophenylporphyrin Protonation. Crucial Role of Hydrogen Bonds
Vladimir B. Sheinin,a@ Olga M. Kulikova,a and Oscar I. Koifmana,b
aG.A. Krestov Institute of Solution Chemistry of the Russian Academy of Sciences, 153045 Ivanovo, Russia
bIvanovo State University of Chemistry and Technology, 153000 Ivanovo, Russia
@Corresponding author E-mail: vbs@isc-ras.ru
DOI: 10.6060/mhc181109s
Macroheterocycles 2018 11(4) 363-370
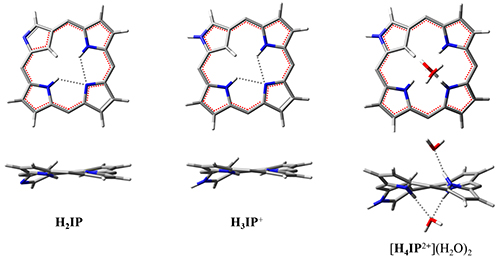
Diprotonation equilibriaof inverted platform of tetraanionic 5,10,15,20-tetrakis(4′-sulfophenyl)-2-aza-21-carbaporphyrin, H2IP(PhSO3H)4, with perchloric acid in water was studied using DFT/B3LYP/6-31++G(d,p) and spectropotentiometric titration methods. In aqueous solution this porphyrinoid exists in the form of NH tautomer H2I(i)P(PhSO3-)4 with an inverted pyrrolenine ring, which is stabilized by bifurcated intramolecular hydrogen bonds between two pyrrole hydrogens and one pyrrolenine nitrogen. Intramolecular hydrogen bonds protect intramolecular hydrogen-bonding sites from intermolecular interactions. For this reason, the external nitrogen atom is protonated first, and then the internal one is protonated. The second proton switches the intramolecular hydrogen bonds to intermolecular hydrogen bonds. Diprotonated platform H4IP2+(PhSO3-)4 has the geometry of elastic 1,3-alternate, which is a molecular and anionic receptor. The equilibrium of the second stage protonation in water is completely shifted to the aquacomplex [H4IP2+(PhSO3-)4](H2O)2, which is formed due to the hydrogen and electrostatic binding of the solvent molecules on both receptor sites. Aquacomplex [H4IP++(PhSO3-)4](H2O)2 is a monomer of linear J-aggregates self-assembly. The driving force of J-aggregates assembly is the formation of stronger anionic complexes as a result of water molecules intermolecular replacement by sulfonate groups of monomers.
| Attachment | Size |
|---|---|
| mhc181109s.pdf | 1.48 MB |
- 1326 reads
- Русский
