Журнал "Макрогетероциклы"
Журнал является форумом специалистов, изучающих макрогетероциклические соединения
Navigation
News
Impact Factor 2021 = 1.200 has been issued by ISI Web of Knowledge (JCR 2021).
Search
ISSN 1998-9539
Spectrophotometrical Study of the Physisorption of Iron(II) Clathrochelates Containing Terminal Phenanthrenyl Group(s) on Carbon Paper
Yan Z. Voloshin,a,b@ Nina V. Chornenka,c Alexander S. Belov,d Sergey A. Grigoriev,e Artem S. Pushkarev,e,f Pierre Millet,g Valery N. Kalinichenko,h Irina G. Belaya,a,d Margarita G. Bugaenko,a,d and Alexey G. Dedova,b
aGubkin Russian State University of Oil and Gas (National Research University), 119991 Moscow, Russia
bKurnakov Institute of General and Inorganic Chemistry of the Russian Academy of Sciences, 119991 Moscow, Russia
cVernadskii Institute of General and Inorganic Chemistry NASU, 03142 Kyiv, Ukraine
dNesmeyanov Institute of Organoelement Compounds of the Russian Academy of Sciences, 119991 Moscow, Russia
eNational Research University “Moscow Power Engineering Institute”, 111250 Moscow, Russia
fNational Research Centre “Kurchatov Institute”, 123182 Moscow, Russia
gInstitut de Chimie Moléculaire et des Matériaux d’Orsay, Université Paris-Sud, 91405 Orsay, France
hSemenov Institute of Chemical Physics, 119991 Moscow, Russia
@Corresponding author E-mail: voloshin@ineos.ac.ru
DOI: 10.6060/mhc181008v
Macroheterocycles 2018 11(4) 449-453
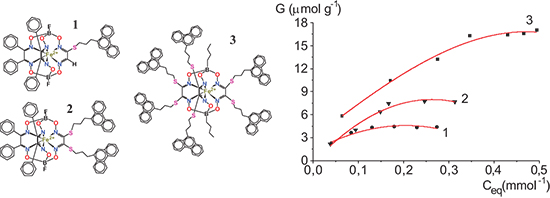
Transition metal clathrochelates form a family of the compounds of great practical interest for the hydrogen evolution reaction in acidic electrolytes. When adequately immobilized on the surface of appropriate carbonaceous substrates, they can find application at the cathode of polymer electrolyte membrane water electrolysis cells. The physisorption of the mono-, di- and hexa-functionalized phenanthrenyl-terminated iron(II) clathrochelates, which were especially designed for their effective immobilization onto carbonaceous substrates, onto a carbon paper of practical interest, was studied by UV-Vis spectrophotometry. It was found that the larger the number of polyaromatic groups per a clathrochelate molecule, the stronger the physisorption is. Despite a larger steric hindrance, the complexes with several polyaromatric groups are prone to stronger physisorption on carbonaceous substrates. The physisorption of Methylene Blue dye, a model compound, was used as reference for comparison.
| Attachment | Size |
|---|---|
| mhc181008v.pdf | 1.03 MB |
- 1307 reads
- Русский
