Журнал "Макрогетероциклы"
Журнал является форумом специалистов, изучающих макрогетероциклические соединения
Navigation
News
Impact Factor 2021 = 1.200 has been issued by ISI Web of Knowledge (JCR 2021).
Search
ISSN 1998-9539
Photophysical Properties and Photochemical Activity of Metal Phthalocyanines Adsorbed on Modified Montmorillonite
Margarita A. Gradova,a@ Irina I. Ostashevskaya,b Oleg V. Gradov,a Anton V. Lobanov,a and Viktor B. Ivanova
aSemenov Institute of Chemical Physics, Russian Academy of Sciences, 119991 Moscow, Russia
bLomonosov Moscow State University, Department of Chemistry, 119991 Moscow, Russia
@Corresponding author E-mail: m.a.gradova@gmail.com
DOI: 10.6060/mhc181001g
Macroheterocycles 2018 11(4) 404-411
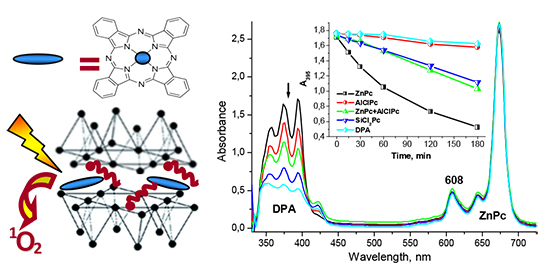
Phthalocyanine metal complexes (MPc) with d0-and d10-metals are known to be promising photosensitizers and photocatalysts due to high molar extinction coefficients within 600–800 nm spectral range and long lifetime of the triplet excited states. However, their wide practical application is strongly limited by their poor solubility in most organic solvents and inherent tendency to form different types of aggregates. In most cases, interchromophore interactions in the aggregates result in the decrease of the triplet state lifetime, and hence, reduce both photodynamic and catalytic activity of the phthalocyanine-based materials. Control upon the aggregation state and photophysical properties of phthalocyanines can be performed after their immobilization on the surface of a solid inert carrier, which prevents macrocycles from aggregation. This paper describes a preparation procedure of heterogeneous photocatalysts based on zinc(II), aluminum(III) and silicon(IV) phthalocyanines adsorbed on the surface of organophilic clay particles composed of a cationic surfactant-modified montmorillonite. The aggregation behavior and photophysical properties of the adsorbed metal complexes are considered in details, and their photocatalytic activity in photosensitized oxidation of a model compound – 9,10-diphenylanthracene under visible light irradiation is compared. The interaction between the adsorbates during adsorption from a solution containing a mixture of the metal phthalocyanine complexes is also described. The crucial role of the axial ligands in regulating the aggregation state, and hence, the photophysical properties and photochemical activity of the phthalocyanine metal complexes in the adsorption layer is established. It was found, that a mixed solvent containing DMF-EtOH-H2O with 1:2:1 volume ratio allows to increase MPc adsorption efficiency up to 95 %, compared to the pure DMF or EtOH solutions, providing only 25 and 35 %, respectively. Hence, the decrease in the solvation capacity of the solvent towards MPc results in a more favorable interaction of the adsorbate with the clay surface. The organophilic clay powder modified with MPc produced a stable transparent suspension after sonication in toluene. In the resulting suspensions ZnPc and SiCl2Pc were found to be stabilized in a fluorescent monomolecular form, while AlClPc was shown to assemble into J-type aggregates through the bridging axial ligands. The simultaneous presence of ZnPc and AlClPc at the clay surface prevents the latter from self-assembly into J-type aggregates, while a successful formation of H-type aggregates was observed in this mixture. For the toluene suspensions containing AlClPc adsorbed on the mineral particle surface, the increase in the amount of both types of aggregates was observed upon storage, while the other metal phthalocyanines in the adsorption layer remained in a monomolecular form. In the case of SiCl2Pc, the reason for the enhanced stability towards aggregation is the steric hindrances produced by two axial ligands which are located on the opposite sides of the macrocycle plane, preventing them from interchromophore interactions. Despite the effective monomolecular form stabilization at the clay surface, SiCl2Pc in toluene suspensions was shown to possess lower photocatalytic activity in the photosensitized oxidation of 9,10-diphenylanthracene, compared to ZnPc. This may be due to the hindered access of the molecular oxygen towards the excited photosensitizer molecule by the axial ligands in the course of the energy transfer process. As expected, AlClPc with the dominating aggregated forms on the clay surface demonstrated the lowest photochemical activity in similar conditions. Therefore, metal phthalocyanine complexes with the unsaturated coordination sphere possess an increased tendency to form supramolecular aggregates via axial ligand-assisted self-assembly, and hence, are not suitable for photocatalytic applications without additional stabilization of their monomolecular form at the organophilic montmorillonite surface.
| Attachment | Size |
|---|---|
| mhc181001g.pdf | 1.46 MB |
- 1753 reads
- Русский
