Журнал "Макрогетероциклы"
Журнал является форумом специалистов, изучающих макрогетероциклические соединения
Navigation
News
Impact Factor 2021 = 1.200 has been issued by ISI Web of Knowledge (JCR 2021).
Search
ISSN 1998-9539
Synthesis and Antiviral Activity of Diaza-18-crown-6 Derivatives with the Fragments of 4-Aminomethylbenzoic and 6-Aminocaproic Acids
Stepan S. Basok,a Anatoliy F. Lutsyuk,a@ Tatyana L. Gridina,b and Alla S. Fedchukc
aA.V. Bogatsky Physico-Chemical Institute, National Academy of Sciences of Ukraine, 65080 Odessa, Ukraine
bOdessa National Medical University, 65026 Odessa, Ukraine
cBPPP Research Center, 65003 Odessa, Ukraine
@Corresponding author E-mail: lutsyuk@ukr.net
DOI: 10.6060/mhc180796l
Macroheterocycles 2018 11(4) 442-448
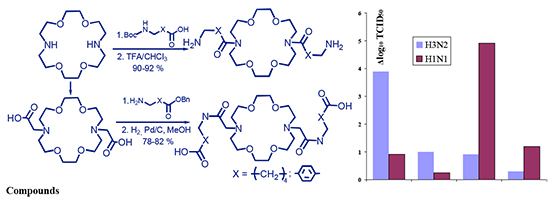
Among the most important properties governing wide spectrum of biological activities of crown ethers are their high lipophilicity, selectivity of the complex formation as well as ability of transporting ions and some neutral molecules across the biological membranes, similarly to the natural ionophores. Modification of diazacrown ethers by introducing fragments of known antiviral agents into their structure can be assumed to yield new products with high antiviral activity. The compounds of a class of protease inhibitors, 4-aminomethylbenzoic and 6-aminocaproic acids are of interest in respect of this. We have synthesized derivatives with free amino and carboxyl groups containing fragments of known antiviral drugs, 4-aminomethylbenzoic and 6-aminocaproic acids, based on the macrocyclic diaza-18-crown-6 platform. The cytotoxicity and antiviral activity of synthesized compounds against of human influenza strains А/Hong Kong/1/68 (H3N2) and А/Puerto Rico/8/34 (H1N1) have been studied. Synthesis of compounds with free amino group (1,10-bis(6-aminohexanoyl)-diaza-18-crown-6 and 1,10-bis(4-aminomethylbenzoyl)-diaza-18-crown-6) have been carried out by interaction of diaza-18-crown-6 with Boc-protected derivatives of 6-aminocaproic and 4-aminomethylbenzoic acids in the presence of DCC and HOBT followed by removal of the Boc-protective group in the intermediate compounds by the action of trifluoroacetic acid. It should be noted that using HOBT allowed us to avoid the formation of collateral bis-N-acylureas, simplify the purification, and increase the total yields of final products to 90–92 %. Acylation of benzyl esters of studied N,N'-dicarboxymethyldiaza-18-crown-6 amino acids was investigated to obtain the compounds with the free carboxyl group, 6,6'-{(7,16-diaza-18-crown-6)-7,16-diylbis[(1-oxoethane-2,1-diyl)imino]} dihexanoic acid and 4,4'-{(7,16-diaza-18-crown-6)-7,16-diylbis[(1-oxoethane-2,1-diyl)iminomethylene]} dibenzoic acid. In this case, using DCC as a condensing agent has resulted the main reaction product to be macrocyclic bis-N-acylureas, and the desired compounds have not been obtained. Therefore, another method of peptide chemistry was used for the acylation of esters of the studied acids – mixed anhydride method with ethyl chloroformate. By this method the benzyl esters of dipeptides with yields of 85–87 % were obtained. Subsequent removal of the benzyl protecting group in these compounds by catalytic hydrogenolysis resulted in desired products with yields of 92–94 %. The toxicity level for all the synthesized compounds was studied on a model using Colpoda steinii infusoria culture and the chorionallantoic membrane cells of chick embryos, and antiviral activity against human influenza strains A/Hong Kong/1/68 (H3N2) and А/Puerto Rico/8/34 (H1N1) in culture of the chorionallantoic membrane cells of chick embryos. Synthesized compounds showed significantly higher level of antiviral activity compared to that of 4-aminomethylbenzoic and 6-aminocaproic acids on both strains of the influenza virus with no cytotoxicity demonstrated in the studied concentrations. The compounds with 6-aminocaproic acid fragments were more active toward both virus strains. It should be noted that the 1,10-bis(6-aminohexanoyl)-diaza-18-crown-6 showed marked activity towards influenza A/Hong Kong/1/68 (H3N2) strain not only at a concentration of 1 mmol/L (Δlog10 TCID50=3.9), but also at a lower dose of 0.5 mmol/L (Δlog10 TCID50=1.17). 6,6'-{(7,16-Diaza-18-crown-6)-7,16-diylbis[(1-oxoethane-2,1-diyl)imino]} dihexanoic acid was the most effective against the strain of the influenza А/Puerto Rico/8/34 (H1N1) virus. It significantly suppressed the reproduction of the virus at a concentration of 0.5 mmol/L (Δlog10 TCID50=4.92). Both compounds demonstrate the level of the reference drug Oseltamivir regarding their influenza activity and are promising compounds for the further research on the in vivo models for the purpose of designing the new antiviral drugs.
| Attachment | Size |
|---|---|
| mhc180796l.pdf | 673.71 KB |
- 1222 reads
- Русский
