Журнал "Макрогетероциклы"
Журнал является форумом специалистов, изучающих макрогетероциклические соединения
Navigation
News
Impact Factor 2021 = 1.200 has been issued by ISI Web of Knowledge (JCR 2021).
Search
ISSN 1998-9539
Proton Exchange between Water Molecules and Intracyclic NH Groups of Dimethyl Ether of Deuteroporphyrin
Alexander L. Stolypko,a and Dmitriy V. Belykhb@
aSyktyvkar State University, 167000 Syktyvkar, Russia
bInstitute of Chemistry, Komi Science Centre, Ural Branch of the Russian Academy of Sciences, 167982 Syktyvkar, Russia
@Corresponding author E-mail: belykh-dv@mail.ru
DOI: 10.6060/mhc180689b
Macroheterocycles 2018 11(4) 383-389
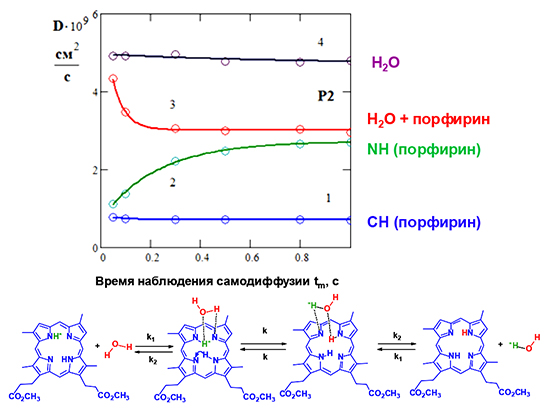
The proton exchange of deuteroporphyrin dimethyl ether internal NH protons with water molecules contained in deuterated chloroform was studied by DOSY. The solution components self-diffusion kinetics for bilateral chemical exchange was calculated in the fast diffusion approximation. It is shown that the measured self-diffusion coefficients of the components D with an increase in the diffusion observation time tm (the interval between the gradient pulses in the DOSY method) exponentially approach the common limit. The limiting values of D depend on the molar ratios of the compounds participating in the exchange. The experimental data obtained for the coefficients of self-diffusion of NH protons and water protons (DNH and DW) in deuterated chloroform, firstly, confirm the observance of the fast exchange condition over the whole range of variation of tm; secondly, the behavior of the measured DNH and DW as a function of tm coincides with the calculated one. This proves that the effect observed is due to the exchange processes between the internal NH-groups of porphyrin and water molecules. The rate constants of the porphyrin internal NH-proton transfer to the water molecule and the inverse process were measured, respectively, kNH≈4.5 sec-1 and kW≈16 sec-1.
| Attachment | Size |
|---|---|
| mhc180689b.pdf | 1.84 MB |
- 1215 reads
- Русский
