Yu. L. Sebyakin,a A. A. Loseva,a@ U. A. Budanova,a and A. V. Lubeshkinb
аMoscow State Technological University, Institute of Fine Chemical Technologies, 119571 Moscow, Russia
bN.D. Zelinsky Institute of Organic Chemistry of Russian Academy of Sciences, 119991 Moscow, Russia
@Corresponding author E-mail: c-221@yandex.ru
DOI: 10.6060/mhc160644s
Macroheterocycles 2016 9(3) 314-319
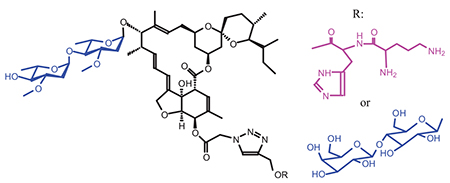
A significant number of macrocyclic drugs are currently on the market, predominantly of natural product origin with complex structures. These drugs have a size of macrocycle more than twelve atoms. Because of their size and complexity they can engage targets through numerous and spatially distributed binding interactions, thereby increasing both binding affinity and selectivity. Furthermore, cyclization provides a degree of structural pre-organization that may reduce the entropy cost of receptor binding as compared to linear analogues. Сyclization has a favorable impact on other essential properties required for drugs, such as membrane permeability, metabolic stability and over all pharmacokinetics. However, significant progress has recently been made, what increased the synthetic tractability of the macrocycles. Most of these are used in the treatment of infections of viral and bacterial origin, helminthiasis or fungi. The current dataset reveals that macrocycles are still predominantly developed for use in oncology and infection, just as registered macrocyclic drugs. Furthermore, there is evidence for positive effects of pharmacophoric nitrogen-containing (hydrazide, amine, amide, pyrrolidone, pyrrolizidine, oxazole, etc.) fragments in the macrolide molecules on identification or intensification of anti-inflammatory, analgesic, antiviral and antimicrobial, fungicidal, anticancer and anti-tumor as well as immunomodulating activities. One of such compounds is ivermectin B1 - semisynthetic macrolide. Ivermectin is macrocyclic lactones derived from the bacterium Streptomyces avermitilis. It is a mixture of 22,23-dihydroavermectin B1. Ivermectin is an avermectin which a group of pentacyclic sixteen-membered lactones (i.e. a macrocyclic lactone disaccharide). It is a broad-spectrum antiparasitic agent. Ivermectin mainly used for humans in the treatment of onchocerciasis, but is also effective against other worm infestations, and some epidermal parasitic skin diseases, including scabies. It kills parasites by interfering with their nervous system and muscle function due to its ability to bind to the glutamate-gated chloride channels. It has a low toxicity for warm-blooded, high stability, the intensity of antiparasitic and broad spectrum of activity, including antiviral activity against various flaviviruses and alphaviruses. There is also information about the anticancer properties of ivermectin. The presence of reactive centers allows using of a series of chemical reactions and synthesizes new derivatives by introducing various groups. In this article, synthesis of macrolides with potential biological activity based on ivermectin derivatives with dipeptide and carbohydrate was described. The basis of synthesis is the reaction of 1,3-dipolar cycloaddition, the main characteristics of which are a high reaction rate, regiospecificity, high yields and mild conditions. This method of “click”-chemistry widely used in different fields of bioorganic chemistry represents the conjugation of two blocks. The first one is propargyl ether of N-(tert-butyloxycarbonyl)-L-ornityl-L-histidine. The second block is azide-containing ivermectin derivative. By similar conjugation of hydrophilic component 1-O-propargyl-4-O-β-D-galactopyranosyl-α-D-glucopyranoside and azide-derivative of ivermectin new macrolide with moiety of carbohydrate was synthesized. New conjugates extend the range of available products and improve their water solubility significantly facilitates biochemical studies to determine the biological activity of the compounds.
References:
