Lyudmila N. Lysenkova,a@1 Oleg Y. Saveljev,b Alexander M. Korolev,a Valery N. Danilenko,c Olga B. Bekker,c Dilara A. Mavletova,c Aleksey A. Vatlin,c Olga A. Omelchuk,a and Andrey E. Shchekotihina@2
aGause Institute of New Antibiotics, 119021 Moscow, Russian Federation
bLomonosov Moscow State University, 119991 Moscow, Russian Federation
cVavilov Institute of General Genetics of Russian Academy of Sciences, 119333 Moscow, Russian Federation
@1Corresponding author E-mail: lyudmil-lys@yandex.ru
@2Corresponding author E-mail: shchekotikhin@mail.ru
DOI: 10.6060/mhc160422s
Macroheterocycles 2016 9(3) 307-313
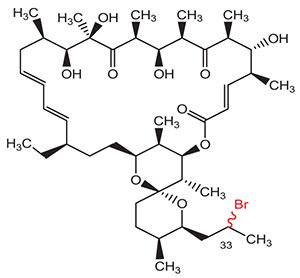
A new derivative of macrolide antibiotic oligomycin A – bromooligomycin 3 has been synthesized. Reaction of 33-O-mesyloligomycin A (2) with tetra-butylammonium bromide or potassium bromide produced a mixture of two diastereomers 33-(R,S)-bromo-33-deoxyoligomycin A (3). The structure was confirmed by NMR spectroscopy and high-resolution mass spectrometry. Antimicrobial activity and physicochemical properties of 33-deoxy-33-bromoligomycin A (3) were described for a mixture of diastereomers.
References:
