Журнал "Макрогетероциклы"
Журнал является форумом специалистов, изучающих макрогетероциклические соединения
Navigation
News
Impact Factor 2021 = 1.200 has been issued by ISI Web of Knowledge (JCR 2021).
Search
ISSN 1998-9539
Terpenylphenol-Chlorin Conjugates in Reaction with Peroxy- Radicals
L. I. Mazaletskaya,a@ N. I. Sheludchenko,a I. S. Khudyaeva,b E. V. Buravlev,b D. V. Belykh,b I. Yu. Chukichevab
aEmanuel Institute of Biochemical Physics, Russian Academy of Sciences, 119334 Moscow, Russian Federation
bInstitute of Chemistry, Komi Scientific Center, Ural Division, Russian Academy of Sciences, 167982 Syktyvkar, Russia
@Corresponding author E-mail: lim@sky.chph.ras.ru
DOI: 10.6060/mhc150973m
Macroheterocycles 2015 8(4) 371-375
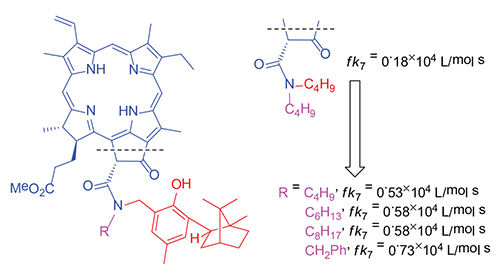
Chlorophyll a and its chemically modified derivatives are biologically active substances which are widely used in medical chemistry. Moreover, there are some data on the possibility of their use as antioxidants. An introduction of terpenylphenolic fragment at the periphery of the macrocycle can increase their own antioxidant activity of the parent molecule. Therefore the compounds combining in their molecules the fragments of terpenylphenol and chlorophyll a derivatives are of a great interest as potential antioxidants. At the present study, we have investigated an antiradical activity of terpenylphenol-chlorin conjugates, and the contribution of each fragment to the antiradical activity of the molecule as a whole was estimated. The classic method based on the oxygen detection uptake during the oxidation was used for antioxidant activity estimation. It was found that the antiradical activity of terpenylphenol-chlorin conjugates with an amide bond between the porphyrin and terpenylphenol fragments is caused by the presence of the terpenylphenol fragment. The conjugates activity appreciably exceeds the chlorophyll derivatives activity and at the same time, significantly is lower than terpenylphenols activity. Higher antiradical activity of conjugates compared with free chlorin macrocycles indicates that, despite the significant loss of activity, the phenolic group retains the ability to interact with free radicals.
| Attachment | Size |
|---|---|
| mhc150973m.pdf | 548.01 KB |
- 1514 reads
- Русский
